Aortic Valve Mechanobiology –
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Health Facilities and Services Review Board
STATE OF ILLINOIS HEALTH FACILITIES AND SERVICES REVIEW BOARD 525 WEST JEFFERSON ST. • SPRINGFIELD, ILLINOIS 62761 •(217) 782-3516 FAX: (217) 785-4111 DOCKET NO: BOARD MEETING: PROJECT NO: September 14, 2021 21-016 PROJECT COST: H-04 FACILITY NAME: CITY: Original: $170,520,604 NorthShore Glenbrook Hospital Glenview TYPE OF PROJECT: Substantive HSA: VII PROJECT DESCRIPTION: The Applicant [NorthShore University HealthSystem] is asking the State Board approve establishment of an open-heart surgery category of service, the addition of 8 cardiac cath labs, and the addition of 6 surgery rooms at Glenbrook Hospital in Glenview, Illinois. The cost of the project is $170,520,604. The expected completion date is December 31, 2024. The purpose of the Illinois Health Facilities Planning Act is to establish a procedure (1) which requires a person establishing, constructing or modifying a health care facility, as herein defined, to have the qualifications, background, character and financial resources to adequately provide a proper service for the community; (2) that promotes the orderly and economic development of health care facilities in the State of Illinois that avoids unnecessary duplication of such facilities; and (3) that promotes planning for and development of health care facilities needed for comprehensive health care especially in areas where the health planning process has identified unmet needs. Cost containment and support for safety net services must continue to be central tenets of the Certificate of Need process. (20 ILCS 3960/2) The Certificate of Need process required under this Act is designed to restrain rising health care costs by preventing unnecessary construction or modification of health care facilities. -
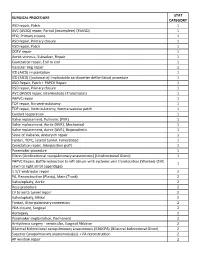
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Transcatheter Mitral and Pulmonary Valve Therapy
Journal of the American College of Cardiology Vol. 53, No. 20, 2009 © 2009 by the American College of Cardiology Foundation ISSN 0735-1097/09/$36.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2008.12.067 FOCUS ISSUE: VALVULAR HEART DISEASE Transcatheter Mitral and Pulmonary Valve Therapy Nicolo Piazza, MD,* Anita Asgar, MD,† Reda Ibrahim, MD,‡ Raoul Bonan, MD‡ Rotterdam, the Netherlands; London, United Kingdom; and Montreal, Quebec, Canada As the percentage of seniors continues to rise in many populations around the world, the already challenging burden of valvular heart disease will become even greater. Unfortunately, a significant proportion of patients with moderate-to-severe valve disease are refused or denied valve surgery based on age and/or accompanying comorbidities. Furthermore, because of advances in pediatric cardiology, the number of adult patients with con- genital heart disease is on the rise and over time, these patients will likely require repeat high-risk surgical pro- cedures. The aim of transcatheter valve therapies is to provide a minimally invasive treatment that is at least as effective as conventional valve surgery and is associated with less morbidity and mortality. The objective of this review was to provide an update on the clinical status, applicability, and limitations of transcatheter mitral and pulmonary valve therapies. (J Am Coll Cardiol 2009;53:1837–51) © 2009 by the American College of Cardiology Foundation The prevalence of moderate-to-severe valvular heart disease ing prevalence of adults with congenital heart disease. It is is highly age-dependent, ranging from an estimated 0.7% in safe to assume that some of these patients will require 18- to 44-year-olds in the U.S. -

(12) United States Patent (10) Patent No.: US 8,187,323 B2 Mortier Et Al
USOO8187323B2 (12) United States Patent (10) Patent No.: US 8,187,323 B2 Mortier et al. (45) Date of Patent: May 29, 2012 (54) VALVE TO MYOCARDIUM TENSION 4,372,293 A 2/1983 Vijil-Rosales MEMBERS DEVICE AND METHOD 4.409,974 A 10, 1983 Freedland 4,536,893 A 8, 1985 Parravicini 4,690,134 A 9/1987 Snyders (75) Inventors: Todd J. Mortier, Minneapolis, MN 4,705,040 A 1 1/1987 Mueller et al. (US); Cyril J. Schweich, Jr., St. Paul, 4,936,857 A 6, 1990 Kulik MN (US) 4,944,753. A 7/1990 Burgess et al. 4,960,424 A 10, 1990 Grooters (73) Assignee: Edwards Lifesciences, LLC, Irvine, CA 4,997.431 A 3, 1991 Isner et al. US 5,104,407 A 4, 1992 Lam et al. (US) 5,106,386 A 4, 1992 Isner et al. 5,131,905 A 7, 1992 Grooters (*) Notice: Subject to any disclaimer, the term of this RE34,021 E 8, 1992 Mueller et al. patent is extended or adjusted under 35 5,169,381 A 12/1992 Snyders U.S.C. 154(b) by 205 days. (Continued) (21) Appl. No.: 09/981,790 FOREIGN PATENT DOCUMENTS (22) Filed: Oct. 19, 2001 DE 36 14292 c 111987 (Continued) (65) Prior Publication Data OTHER PUBLICATIONS US 20O2/OO2908O A1 Mar. 7, 2002 Dickstein et al., “Heart Reduction Surgery: An Analysis of the Impact Related U.S. Application Data on Cardiac Function.” The Journal of Thoracic and Cardiovascular (63) Continuation of application No. 08/992.316, filed on Surgery, vol. -

Surgery for Acquired Heart Disease
View metadata, citation and similar papers at core.ac.uk brought to you byCORE provided by Elsevier - Publisher Connector SURGERY FOR ACQUIRED HEART DISEASE EARLY RESULTS WITH PARTIAL LEFT VENTRICULECTOMY Patrick M. McCarthy, MD a Objective: We sought to determine the role of partial left ventriculectomy in Randall C. Starling, MD b patients with dilated cardiomyopathy. Methods: Since May 1996 we have James Wong, MBBS, PhD b performed partial left ventriculectomy in 53 patients, primarily (94%) in Gregory M. Scalia, MBBS b heart transplant candidates. The mean age of the patients was 53 years Tiffany Buda, RN a Rita L. Vargo, MSN, RN a (range 17 to 72 years); 60% were in class IV and 40% in class III. Marlene Goormastic, MPH c Preoperatively, 51 patients were thought to have idiopathic dilated cardio- James D. Thomas, MD b myopathy, one familial cardiomyopathy, and one valvular cardiomyopathy. Nicholas G. Smedira, MD a As our experience accrued we increased the extent of left ventriculectomy James B. Young, MD b and more complex mitral valve repairs. For two patients mitral valve replacement was performed. For 51 patients the anterior and posterior mitral valve leaflets were approximated (Alfieri repair); 47 patients also had ring posterior annuloplasty. In 27 patients (5!%) one or both papillary muscles were divided, additional left ventricular wall was resected, and the papillary muscle heads were reimplanted. Results: Echocardiography showed a significant decrease in left ventricular dimensions after resection (8.3 cm to 5.8 cm), reduction in mitral regurgitation (2.8+ to 0), and increase in forward ejection fraction (15.7% to 32.7%). -

Reduction Ventriculoplasty for Dilated Cardiomyopathy : the Batista Procedure Shahram Salemy Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1999 Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure Shahram Salemy Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Salemy, Shahram, "Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure" (1999). Yale Medicine Thesis Digital Library. 3123. http://elischolar.library.yale.edu/ymtdl/3123 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. SlDDCITOM VENTRICULOPIASTy FOR DILATED CARDIOMYOPATHY THE BATISTA PROCEDURE W«M * (e,yx»> ShaLramSalemy YALE DNIVERSriY YALE UNIVERSITY CUSHING/WHITNEY MEDICAL LIBRARY Permission to photocopy or microfilm processing of this thesis for the purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work or otherwise placing it in the public domain, and the author reserves all rights of ownership guaranteed under common law protection of unpublished manuscripts. Signature of Author Date REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE Shahram Salemy B.S., George Tellides M.D., Ph.D., and John A. Elefteriades M.D. February 5, 1999 r 113 f'Uh (e(e.cl 0 REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE. -

Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G
ORIGINAL ARTICLES: CONGENITAL HEART SURGERY CONGENITAL HEART SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal. CONGENITAL HEART Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G. Co-Vu, MD,* Salil Ginde, MD,* Peter J. Bartz, MD, Peter C. Frommelt, MD, James S. Tweddell, MD, and Michael G. Earing, MD Department of Pediatrics, Division of Pediatric Cardiology, and Department of Internal Medicine, Division of Cardiovascular Medicine, and Department of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, Wisconsin Background. After the arterial switch operation (ASO) score increased at an average rate of 0.08 per year over for transposition of the great arteries (TGA), the native time after ASO. Freedom from neoaortic root dilation at pulmonary root and valve function in the systemic posi- 1, 5, 10, and 15 years after ASO was 84%, 67%, 47%, and tion, and the long-term risk for neoaortic root dilation 32%, respectively. Risk factors for root dilation include -pre ,(0.003 ؍ and valve regurgitation is currently undefined. The aim history of double-outlet right ventricle (p and length of ,(0.01 ؍ of this study was to determine the prevalence and pro- vious pulmonary artery banding (p Neoaortic valve regurgitation of at .(0.04 ؍ gression of neoaortic root dilation and neoaortic valve follow-up (p regurgitation in patients with TGA repaired with the least moderate degree was present in 14%. -

Heart Valve Surgery Factsheet
Heart information Heart valve surgery Contents Page 1 What are heart valves? Page 3 What is heart valve surgery? Page 3 Valve repair Page 4 Valve replacement Page 4 What happens during heart valve surgery? Page 5 Why do I need heart valve surgery? Page 6 What will happen after heart valve surgery? Page 6 Will I feel pain after the operation? Page 6 How long will I have to stay in hospital? Page 7 How long will it take to recover from heart valve surgery? Page 7 When can I start eating again? Page 8 What should I be eating? Page 8 What if I am constipated? Page 8 How active can I be? Page 9 When can I be more physically active? Page 9 When can I have sex? Page 10 Why am I so tired? Page 10 Why do I feel great one day, but dreadful the next? Page 10 When can I return to work? Page 10 Palpitations Page 11 Heart attack Page 12 Endocarditis Page 12 Will I need to take medicine? Page 13 Important things to remember about anti-coagulant medicine Page 14 Warfarin and food Page 15 What should I talk to my doctor about? Page 16 Glossary What are heart valves? For more Heart valves are like one-way doors that control the direction of blood flow between the four chambers of the heart. There are information two upper chambers (the atria) and two lower chambers (the on this topic ventricles) (see the diagram on page 2). The atria receive blood please call from the body and pump it into the ventricles. -

Medicare National Coverage Determinations Manual, Part 1
Medicare National Coverage Determinations Manual Chapter 1, Part 1 (Sections 10 – 80.12) Coverage Determinations Table of Contents (Rev. 10838, 06-08-21) Transmittals for Chapter 1, Part 1 Foreword - Purpose for National Coverage Determinations (NCD) Manual 10 - Anesthesia and Pain Management 10.1 - Use of Visual Tests Prior to and General Anesthesia During Cataract Surgery 10.2 - Transcutaneous Electrical Nerve Stimulation (TENS) for Acute Post- Operative Pain 10.3 - Inpatient Hospital Pain Rehabilitation Programs 10.4 - Outpatient Hospital Pain Rehabilitation Programs 10.5 - Autogenous Epidural Blood Graft 10.6 - Anesthesia in Cardiac Pacemaker Surgery 20 - Cardiovascular System 20.1 - Vertebral Artery Surgery 20.2 - Extracranial - Intracranial (EC-IC) Arterial Bypass Surgery 20.3 - Thoracic Duct Drainage (TDD) in Renal Transplants 20.4 – Implantable Cardioverter Defibrillators (ICDs) 20.5 - Extracorporeal Immunoadsorption (ECI) Using Protein A Columns 20.6 - Transmyocardial Revascularization (TMR) 20.7 - Percutaneous Transluminal Angioplasty (PTA) (Various Effective Dates Below) 20.8 - Cardiac Pacemakers (Various Effective Dates Below) 20.8.1 - Cardiac Pacemaker Evaluation Services 20.8.1.1 - Transtelephonic Monitoring of Cardiac Pacemakers 20.8.2 - Self-Contained Pacemaker Monitors 20.8.3 – Single Chamber and Dual Chamber Permanent Cardiac Pacemakers 20.8.4 Leadless Pacemakers 20.9 - Artificial Hearts And Related Devices – (Various Effective Dates Below) 20.9.1 - Ventricular Assist Devices (Various Effective Dates Below) 20.10 - Cardiac -

Curriculum Vitae Takahiro Shiota, MD, Phd, FACC, FESC, FASE, FAHA
1 Curriculum Vitae Takahiro Shiota, MD, PhD, FACC, FESC, FASE, FAHA Office Address: Cedars-Sinai Medical Center Heart Institute 127 S. San Vicente Blvd., A3411 Los Angeles, CA 90048 (310) 423-6889 Office Email: [email protected] EDUCATION: 1991 Ph.D. in Cardiology. Faculty of Medicine, University of Tokyo, Tokyo, Japan 1977-1983 M.D. Faculty of Medicine, University of Tokyo, Tokyo, Japan 1972-1976 B.S. in Physics. Faculty of Science, University of Tokyo, Tokyo, Japan LICENSURE AND CERTIFICATION National Board of Echocardiography (#2000-252) California Medical License (#000015) Ohio Medical License (#35. 080318) ECFMG (#0-576-045-9) Japanese Medical License (#274951) PROFESSIONAL EXPERIENCE 1/2009-present Associate Director Division of Noninvasive Cardiology Cedars-Sinai Heart Institute Los Angeles, CA 12/2001-12/2008 Clinical Staff Department of Cardiovascular Medicine Cleveland Clinic, Cleveland, OH 7/1999-11/2001 Advanced Cardiac Department of Cardiovascular Medicine Imaging Fellow Cleveland Clinic, Cleveland, OH 9/1997- 6/1999 Project Staff Department of Cardiovascular Medicine 2 Cleveland Clinic, Cleveland, OH 8/1992- 8/1997 Research Director Cardiac Imaging Laboratory, Clinical Care Center for Congenital Heart Disease, Oregon Health Sciences University, Portland, OR PROFESSIONAL ACTIVITIES: Academic Appointment 7/2009-present Professor of Medicine, Department of Medicine, Cedars-Sinai, Los Angeles, CA 8/2008-present Clinical Professor of Medicine, David Geffen School of Medicine at UCLA 7/2007-12/2008 Professor of Medicine, Cleveland -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

A Focus on Valve-Sparing Ascending Aortic Aneurysm Repair Newyork
ADVANCES IN CARDIOLOGY, INTERVENTIONAL CARDIOLOGY, AND CARDIOVASCULAR SURGERY Affiliated with Columbia University College of Physicians and Surgeons and Weill Cornell Medical College A Focus on Valve-Sparing NOVEMBER/DECEMBER 2014 Ascending Aortic Aneurysm Repair Emile A. Bacha, MD The most frequent location for aneurysms in the Chief, Division of Cardiac, chest occurs in the ascending aorta – and these Thoracic and Vascular Surgery aneurysms are often associated with either aortic NewYork-Presbyterian/Columbia stenosis or aortic insufficiency, especially when the University Medical Center aneurysm involves a bicuspid aortic valve. Director, Congenital and Pediatric Cardiac Surgery “We know that patients who have enlarged NewYork-Presbyterian Hospital aortas or aneurysms of the ascending aorta are at [email protected] great risk for one of two major life-threatening events: an aortic rupture or an aortic dissection,” Allan Schwartz, MD says Leonard N. Girardi, MD, Director of Chief, Division of Cardiology Thoracic Aortic Surgery in the Department of NewYork-Presbyterian/Columbia Cardiothoracic Surgery, NewYork-Presbyterian/ University Medical Center Weill Cornell Medical Center. “Dissection of the Valve-sparing ascending aortic aneurysm repair [email protected] inner lining of the wall of the blood vessel can also lead to rupture or other complications down last 15 years, the Aortic Surgery Program at Weill O. Wayne Isom, MD the line. For example, as the tear extends it may Cornell has been aggressively pursuing the devel- Cardiothoracic Surgeon-in-Chief NewYork-Presbyterian/ affect the vessels that supply the brain or the opment of a procedure that would enable surgeons Weill Cornell Medical Center coronary arteries or cause tremendous damage to to spare the patient’s native valve.