Heart Failure in Aortic Regurgitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
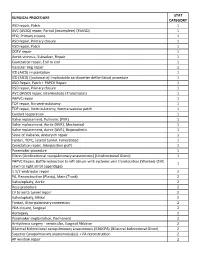
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Surgery for Acquired Heart Disease
View metadata, citation and similar papers at core.ac.uk brought to you byCORE provided by Elsevier - Publisher Connector SURGERY FOR ACQUIRED HEART DISEASE EARLY RESULTS WITH PARTIAL LEFT VENTRICULECTOMY Patrick M. McCarthy, MD a Objective: We sought to determine the role of partial left ventriculectomy in Randall C. Starling, MD b patients with dilated cardiomyopathy. Methods: Since May 1996 we have James Wong, MBBS, PhD b performed partial left ventriculectomy in 53 patients, primarily (94%) in Gregory M. Scalia, MBBS b heart transplant candidates. The mean age of the patients was 53 years Tiffany Buda, RN a Rita L. Vargo, MSN, RN a (range 17 to 72 years); 60% were in class IV and 40% in class III. Marlene Goormastic, MPH c Preoperatively, 51 patients were thought to have idiopathic dilated cardio- James D. Thomas, MD b myopathy, one familial cardiomyopathy, and one valvular cardiomyopathy. Nicholas G. Smedira, MD a As our experience accrued we increased the extent of left ventriculectomy James B. Young, MD b and more complex mitral valve repairs. For two patients mitral valve replacement was performed. For 51 patients the anterior and posterior mitral valve leaflets were approximated (Alfieri repair); 47 patients also had ring posterior annuloplasty. In 27 patients (5!%) one or both papillary muscles were divided, additional left ventricular wall was resected, and the papillary muscle heads were reimplanted. Results: Echocardiography showed a significant decrease in left ventricular dimensions after resection (8.3 cm to 5.8 cm), reduction in mitral regurgitation (2.8+ to 0), and increase in forward ejection fraction (15.7% to 32.7%). -

Reduction Ventriculoplasty for Dilated Cardiomyopathy : the Batista Procedure Shahram Salemy Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1999 Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure Shahram Salemy Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Salemy, Shahram, "Reduction ventriculoplasty for dilated cardiomyopathy : the Batista procedure" (1999). Yale Medicine Thesis Digital Library. 3123. http://elischolar.library.yale.edu/ymtdl/3123 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. SlDDCITOM VENTRICULOPIASTy FOR DILATED CARDIOMYOPATHY THE BATISTA PROCEDURE W«M * (e,yx»> ShaLramSalemy YALE DNIVERSriY YALE UNIVERSITY CUSHING/WHITNEY MEDICAL LIBRARY Permission to photocopy or microfilm processing of this thesis for the purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work or otherwise placing it in the public domain, and the author reserves all rights of ownership guaranteed under common law protection of unpublished manuscripts. Signature of Author Date REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE Shahram Salemy B.S., George Tellides M.D., Ph.D., and John A. Elefteriades M.D. February 5, 1999 r 113 f'Uh (e(e.cl 0 REDUCTION VENTRICULOPLASTY FOR DILATED CARDIOMYOPATHY: THE BATISTA PROCEDURE. -

Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G
ORIGINAL ARTICLES: CONGENITAL HEART SURGERY CONGENITAL HEART SURGERY: The Annals of Thoracic Surgery CME Program is located online at http://cme.ctsnetjournals.org. To take the CME activity related to this article, you must have either an STS member or an individual non-member subscription to the journal. CONGENITAL HEART Long-Term Outcomes of the Neoaorta After Arterial Switch Operation for Transposition of the Great Arteries Jennifer G. Co-Vu, MD,* Salil Ginde, MD,* Peter J. Bartz, MD, Peter C. Frommelt, MD, James S. Tweddell, MD, and Michael G. Earing, MD Department of Pediatrics, Division of Pediatric Cardiology, and Department of Internal Medicine, Division of Cardiovascular Medicine, and Department of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, Wisconsin Background. After the arterial switch operation (ASO) score increased at an average rate of 0.08 per year over for transposition of the great arteries (TGA), the native time after ASO. Freedom from neoaortic root dilation at pulmonary root and valve function in the systemic posi- 1, 5, 10, and 15 years after ASO was 84%, 67%, 47%, and tion, and the long-term risk for neoaortic root dilation 32%, respectively. Risk factors for root dilation include -pre ,(0.003 ؍ and valve regurgitation is currently undefined. The aim history of double-outlet right ventricle (p and length of ,(0.01 ؍ of this study was to determine the prevalence and pro- vious pulmonary artery banding (p Neoaortic valve regurgitation of at .(0.04 ؍ gression of neoaortic root dilation and neoaortic valve follow-up (p regurgitation in patients with TGA repaired with the least moderate degree was present in 14%. -

Curriculum Vitae Takahiro Shiota, MD, Phd, FACC, FESC, FASE, FAHA
1 Curriculum Vitae Takahiro Shiota, MD, PhD, FACC, FESC, FASE, FAHA Office Address: Cedars-Sinai Medical Center Heart Institute 127 S. San Vicente Blvd., A3411 Los Angeles, CA 90048 (310) 423-6889 Office Email: [email protected] EDUCATION: 1991 Ph.D. in Cardiology. Faculty of Medicine, University of Tokyo, Tokyo, Japan 1977-1983 M.D. Faculty of Medicine, University of Tokyo, Tokyo, Japan 1972-1976 B.S. in Physics. Faculty of Science, University of Tokyo, Tokyo, Japan LICENSURE AND CERTIFICATION National Board of Echocardiography (#2000-252) California Medical License (#000015) Ohio Medical License (#35. 080318) ECFMG (#0-576-045-9) Japanese Medical License (#274951) PROFESSIONAL EXPERIENCE 1/2009-present Associate Director Division of Noninvasive Cardiology Cedars-Sinai Heart Institute Los Angeles, CA 12/2001-12/2008 Clinical Staff Department of Cardiovascular Medicine Cleveland Clinic, Cleveland, OH 7/1999-11/2001 Advanced Cardiac Department of Cardiovascular Medicine Imaging Fellow Cleveland Clinic, Cleveland, OH 9/1997- 6/1999 Project Staff Department of Cardiovascular Medicine 2 Cleveland Clinic, Cleveland, OH 8/1992- 8/1997 Research Director Cardiac Imaging Laboratory, Clinical Care Center for Congenital Heart Disease, Oregon Health Sciences University, Portland, OR PROFESSIONAL ACTIVITIES: Academic Appointment 7/2009-present Professor of Medicine, Department of Medicine, Cedars-Sinai, Los Angeles, CA 8/2008-present Clinical Professor of Medicine, David Geffen School of Medicine at UCLA 7/2007-12/2008 Professor of Medicine, Cleveland -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

A Focus on Valve-Sparing Ascending Aortic Aneurysm Repair Newyork
ADVANCES IN CARDIOLOGY, INTERVENTIONAL CARDIOLOGY, AND CARDIOVASCULAR SURGERY Affiliated with Columbia University College of Physicians and Surgeons and Weill Cornell Medical College A Focus on Valve-Sparing NOVEMBER/DECEMBER 2014 Ascending Aortic Aneurysm Repair Emile A. Bacha, MD The most frequent location for aneurysms in the Chief, Division of Cardiac, chest occurs in the ascending aorta – and these Thoracic and Vascular Surgery aneurysms are often associated with either aortic NewYork-Presbyterian/Columbia stenosis or aortic insufficiency, especially when the University Medical Center aneurysm involves a bicuspid aortic valve. Director, Congenital and Pediatric Cardiac Surgery “We know that patients who have enlarged NewYork-Presbyterian Hospital aortas or aneurysms of the ascending aorta are at [email protected] great risk for one of two major life-threatening events: an aortic rupture or an aortic dissection,” Allan Schwartz, MD says Leonard N. Girardi, MD, Director of Chief, Division of Cardiology Thoracic Aortic Surgery in the Department of NewYork-Presbyterian/Columbia Cardiothoracic Surgery, NewYork-Presbyterian/ University Medical Center Weill Cornell Medical Center. “Dissection of the Valve-sparing ascending aortic aneurysm repair [email protected] inner lining of the wall of the blood vessel can also lead to rupture or other complications down last 15 years, the Aortic Surgery Program at Weill O. Wayne Isom, MD the line. For example, as the tear extends it may Cornell has been aggressively pursuing the devel- Cardiothoracic Surgeon-in-Chief NewYork-Presbyterian/ affect the vessels that supply the brain or the opment of a procedure that would enable surgeons Weill Cornell Medical Center coronary arteries or cause tremendous damage to to spare the patient’s native valve. -

The Ross Inclusion Dacron Graft
551 Masters of Cardiothoracic Surgery The Ross inclusion Dacron graft Jama Jahanyar, Daniel E. Muñoz, Saadallah Tamer, Gebrine El Khoury, Laurent de Kerchove Department of Cardiovascular & Thoracic Surgery, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain (UCL), Brussels, Belgium Correspondence to: Laurent de Kerchove, MD, PhD. Department of Cardiovascular & Thoracic Surgery, Cliniques Universitaires Saint-Luc, Avenue Hippocrate 10, 1200 Brussels, Belgium. Email: [email protected]. Submitted Aug 04, 2020. Accepted for publication May 05, 2021. doi: 10.21037/acs-2020-rp-17 View this article at: http://dx.doi.org/10.21037/acs-2020-rp-17 Clinical vignette The tip of a hemostat grabs the right ventricular muscle in the lower triangle of the anterior pulmonary commissure. We present the case of a twenty-eight-year-old male with The hemostat tip is then pushed through the anterior severe aortic stenosis and mean gradient across the aortic wall of the right ventricular outflow tract (RVOT), and valve (AV) of 52 mmHg, due to a calcified congenital countertraction is provided by the surgeon’s hand. Starting unicuspid AV. The patient was asymptomatic, without at the point of RVOT muscle penetration, the pulmonary significant past medical history, and clean coronaries. autograft is excised circumferentially, 3–4 mm proximal Due to extensive AV calcifications, AV repair was not to the pulmonary valve, avoiding injury to the first septal feasible, and Ross procedure was indicated. Echocardiography branch of the left anterior descending artery. Cardioplegia identified a dilated aortic annulus of 28 mm; the aortic is given to identify septal bleeders and hemostasis achieved. -

NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures Measure Number: PCS‐001‐09 Measure Title: Participation in a national database for pediatric and congenital heart surgery Description: Participation in at least one multi‐center, standardized data collection and feedback program that provides benchmarking of the physician’s data relative to national and regional programs and uses process and outcome measures. Participation is defined as submission of all congenital and pediatric operations performed to the database. Numerator Statement: Whether or not there is participation in at least one multi‐center data collection and feedback program. Denominator Statement: N/A Level of Analysis: Group of clinicians, Facility, Integrated delivery system, health plan, community/population Data Source: Electronic Health/Medical Record, Electronic Clinical Database (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Clinical Registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Claims, Paper Medical Record Measure Developer: Society of Thoracic Surgeons Type of Endorsement: Recommended for Time‐Limited Endorsement (Steering Committee Vote, Yes‐9, No‐0, Abstain‐0) Attachments: “STS Attachment: STS Procedure Code Definitions” Meas# / Title/ Steering Committee Evaluation and Recommendation (Owner) PCS-001-09 Recommendation: Time-Limited Endorsement Yes-9; No-0; Abstain-0 Participatio n in a Final Measure Evaluation Ratings: I: Y-9; N-0 S: H-4; M-4; L-1 U: H-9; M-0; L-0 national F: H-7; M-2; L-0 database for Discussion: pediatric I: The Steering Committee agreed that this measure is important to measure and report. and By reporting through a database, it is possible to identify potential quality issues and congenital provide benchmarks. -

Heart Valve Disease
Treatment Guide Heart Valve Disease Heart valve disease refers to any of several condi- TABLE OF CONTENTS tions that prevent one or more of the valves in the What causes valve disease? .................................. 2 heart from functioning adequately to assure prop- er circulation. Left untreated, heart valve disease What are the symptoms of heart valve disease? ....... 5 can reduce quality of life and become life-threat- How is valve disease diagnosed? ............................ 6 ening. In many cases, heart valves can be surgi- What treatments are available? .............................. 8 cally repaired or replaced, restoring normal func- What are the types of valve surgery? ...................... 9 tion and allowing a return to normal activities. What can I expect before and after surgery? .......... 13 Cleveland Clinic’s Sydell and Arnold Miller How can I protect my heart valves? ...................... 17 Family Heart & Vascular Institute is one of the largest centers in the country for the diagnosis and treatment of heart valve disease. The decision to prescribe medical treatment or proceed with USING THIS GUIDE surgical repair or replacement is based on the Please use this guide as a resource as you examine your type of heart valve disease you have, the severity treatment options. Remember, it is every patient’s right of damage, your age and your medical history. to ask questions, and to seek a second opinion. To make an appointment with a heart valve specialist at Cleveland Clinic, call 216.444.6697. CLEVELAND CLINIC | HEART VALVE DISEASE TREATMENT GUIDE About Valve Disease The heart valves How the Valves Work Heart valve disease means one of the heart valves isn’t working properly because The heart has four valves — one for of valvular stenosis (narrowing of the valves) or valvular insufficiency (“leaky” valve). -

Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures Lars G
Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures Lars G. Svensson, David H. Adams, Robert O. Bonow, Nicholas T. Kouchoukos, D. Craig Miller, Patrick T. O'Gara, David M. Shahian, Hartzell V. Schaff, Cary W. Akins, Joseph E. Bavaria, Eugene H. Blackstone, Tirone E. David, Nimesh D. Desai, Todd M. Dewey, Richard S. D'Agostino, Thomas G. Gleason, Katherine B. Harrington, Susheel Kodali, Samir Kapadia, Martin B. Leon, Brian Lima, Bruce W. Lytle, Michael J. Mack, Michael Reardon, T. Brett Reece, G. Russell Reiss, Eric E. Roselli, Craig R. Smith, Vinod H. Thourani, E. Murat Tuzcu, John Webb and Mathew R. Williams Ann Thorac Surg 2013;95:1-66 DOI: 10.1016/j.athoracsur.2013.01.083 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://ats.ctsnetjournals.org/cgi/content/full/95/6_Supplement/S1 The Annals of Thoracic Surgery is the official journal of The Society of Thoracic Surgeons and the Southern Thoracic Surgical Association. Copyright © 2013 by The Society of Thoracic Surgeons. Print ISSN: 0003-4975; eISSN: 1552-6259. Downloaded from ats.ctsnetjournals.org by on May 28, 2013 SPECIAL REPORT Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures Writing Committee Members: Lars G. Svensson, MD, PhD (Chair), David H. Adams, MD (Vice-Chair), Robert O. Bonow, MD (Vice-Chair), Nicholas T. Kouchoukos, MD (Vice-Chair), D. Craig Miller, MD (Vice-Chair), Patrick T. O’Gara, MD (Vice-Chair), David M. Shahian, MD (Vice-Chair), Hartzell V. Schaff, MD (Vice-Chair), Cary W. -

Distribution of Heart Disease in a Multicentre Paediatric Cardiac Registry
TABLE 1: Geometrical comparison Volume 10 • Number 1 LT (n=7) EC (n=3) Summer 2013 T1 T2 Normalised Change T1 T2 Normalised Change Body surface area (m2) 0.9±0.2 1.4±0.2 0.8±0.1 1.4±0.3 Age at CMR (years) 8.7±3.4 13.5±1.9 8.7±2.1 12.7±1.5 Mean diameter (mm) FP 14.9±3.8 18.4±4.7 5.4±2.1 15.1±0.3 15.8±0.6 0.8±0.7 SVC 11.7±3.4 15.0±5.4 5.2±5.3 12.9±0.2 14.5±1.7 2.0±1.3 dAo 10.3±1.5 13.6±1.8 5.2±2.0 11.7±0.5 11.8±1.0 0.3±0.9 LPA 10.6±3.0 11.7±3.0 1.6±3.6 10.3±2.1 11.2±5.2 0.3±4.8 RPA 10.9±3.9 14.4±3.3 5.2±3.0 11.1±2.8 11.3±4.4 -0.3±2.2 Diameter at PA branching (mm) 16.4±5.7 18.9±9.0 3.8±6.2 15.6±1.7 15.1±1.8 -0.6±0.1 FP volume (cm3) 16.1±7.6 29.4±15.7 20.9±14.8 13.0±1.9 16.8±3.0 5.4±1.1 Mean Nakata index (mm2/m2) 207.5±52.6 205.2±45.4 237.7±99.4 154.4±60.6 Mean McGoon ration 2.1±0.4 1.9±0.3 1.8±0.3 1.9±0.7 TABLE 2: Haemodynamic metrics for the LT group Cardiac index (L/min/m2) T1 2.8±0.6 T2 2.8±0.4 TCPC resistance (WU) S1 (T1 geometry, T1 fl ow) 0.5±0.3 S2 (T2 geometry, T2 fl ow) 0.5±0.4 S3 (T1 geometry, T2 fl ow) 0.7±0.8 Serial increase (S2 - S1) 20% Growth absence (S3 - S1) 57% Results are shown as mean ± standard deviation.