HIGHLIGHTS of THIS ISSUE This Listing Does Not Affect the Legal Status of Any Document Published in This Issue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of Pharmacology and Experimental Therapeutics
Journal of Pharmacology and Experimental Therapeutics Molecular Determinants of Ligand Selectivity for the Human Multidrug And Toxin Extrusion Proteins, MATE1 and MATE-2K Bethzaida Astorga, Sean Ekins, Mark Morales and Stephen H Wright Department of Physiology, University of Arizona, Tucson, AZ 85724, USA (B.A., M.M., and S.H.W.) Collaborations in Chemistry, 5616 Hilltop Needmore Road, Fuquay-Varina NC 27526, USA (S.E.) Supplemental Table 1. Compounds selected by the common features pharmacophore after searching a database of 2690 FDA approved compounds (www.collaborativedrug.com). FitValue Common Name Indication 3.93897 PYRIMETHAMINE Antimalarial 3.3167 naloxone Antidote Naloxone Hydrochloride 3.27622 DEXMEDETOMIDINE Anxiolytic 3.2407 Chlordantoin Antifungal 3.1776 NALORPHINE Antidote Nalorphine Hydrochloride 3.15108 Perfosfamide Antineoplastic 3.11759 Cinchonidine Sulfate Antimalarial Cinchonidine 3.10352 Cinchonine Sulfate Antimalarial Cinchonine 3.07469 METHOHEXITAL Anesthetic 3.06799 PROGUANIL Antimalarial PROGUANIL HYDROCHLORIDE 100MG 3.05018 TOPIRAMATE Anticonvulsant 3.04366 MIDODRINE Antihypotensive Midodrine Hydrochloride 2.98558 Chlorbetamide Antiamebic 2.98463 TRIMETHOPRIM Antibiotic Antibacterial 2.98457 ZILEUTON Antiinflammatory 2.94205 AMINOMETRADINE Diuretic 2.89284 SCOPOLAMINE Antispasmodic ScopolamineHydrobromide 2.88791 ARTICAINE Anesthetic 2.84534 RITODRINE Tocolytic 2.82357 MITOBRONITOL Antineoplastic Mitolactol 2.81033 LORAZEPAM Anxiolytic 2.74943 ETHOHEXADIOL Insecticide 2.64902 METHOXAMINE Antihypotensive Methoxamine -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

University Microfilms
INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You w ill find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

The Effect of the Roots of Empathy Program on the Use of Psychotropic Medications Among Youth in Manitoba
The effect of the Roots of Empathy program on the use of psychotropic medications among youth in Manitoba by Lindsey Dahl A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfilment of the requirements of the degree of MASTER OF SCIENCE Department of Community Health Sciences, Rady Faculty of Health Sciences, Max Rady College of Medicine University of Manitoba Winnipeg Copyright © 2017 by Lindsey Dahl Abstract Background: Psychotropic medications prescriptions to youth have increased. Roots of Empathy (ROE) is a social and emotional learning program that may influence the use of psychotropic medication. Methods: Administrative data was analyzed in a matched sample of children who received ROE during 2002/03 to 2012/13. Kaplan-Meier survival curves and Cox proportional hazard models were used to estimate the association between ROE and psychotropic medication dispensations. Results: Few significant differences were observed. Children who received ROE in kindergarten to grade 3 had a lower adjusted hazard for an anxiolytic dispensation. Children who received ROE in grade 7 to 8 had a higher hazard for an antipsychotic dispensation. Males who received the program had an increased hazard for an antipsychotic dispensation. Conclusion: There was no consistent differences in the likelihood of being dispensed a psychotropic medication between children who received ROE and children who did not in Manitoba. ii Acknowledgments I would like to first thank my thesis advisor Dr. Randy Fransoo of the Department of Community Health Sciences, Rady Faculty of Health Sciences, Max Rady College of Medicine at the University of Manitoba. Right from our initial meeting, Dr. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Col.V.MISSION of the EUROPEAN COMMUNITIES
COl.V.MISSION OF THE EUROPEAN COMMUNITIES SEC(90) 1985 final· Brussels, 29 October 1990 Proposa I for a COUNCIL DIRECTIVE on the approximation of the laws of the Member States relat.ing to cosmetic products -2- EXPLANATORY UEUORANDUM 1. In the context of a people's Europe, the Commission attaches great Importance to simplifying and clarifying Community law so as to make It clearer and more accessible to the ordinary citizen, thus giving hIm new opportunItIes and the chance to make use of the spec if i c rights It gives him. This aim cannot be achieved so long as numerous provisions that have been amended several times, often QUite substantially, remain scattered, so that they must be sought partly In the original Instrument and partly In later amending ones. Considerable research work, comparing many different Instruments, Is thus needed to Identify the current rules. For this reason a consot tdatton of rules that have freQuently been amended Is essential If Community law Is to be clear and transparent. 2. In Its resolution of 26 November 1974 concerning consolidation of Its acts (1), the Council recommended that those of Its acts which have been amended several times be assembled Into a single text. It stressed that, In the Interests of legal certainty, a genuine legislative consolidation, Involving the repeal of earlier acts, should wherever possible be effected (as Is being done In this case). it conseQuently Invited the Commission to let it have proposals for consol !dation and undertook ·to examine them "as Quickly as possible, whltout bringing Into QUestion, during that consol ldatlon, the substantive solutions contained In the consol !dated texts". -

Assessment of Molecular Action of Direct Gating and Allosteric Modulatory Effects of Carisoprodol (Somartm) on GABA a Receptors
Graduate Theses, Dissertations, and Problem Reports 2015 Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors Manoj Kumar Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Kumar, Manoj, "Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors" (2015). Graduate Theses, Dissertations, and Problem Reports. 6022. https://researchrepository.wvu.edu/etd/6022 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. ASSESSMENT OF MOLECULAR ACTION OF DIRECT GATING AND ALLOSTERIC MODULATORY EFFECTS OF MEPROBAMATE (MILTOWN®) ON GABAA RECEPTORS Manish Kumar, MD, MS Dissertation submitted to the School of Pharmacy at West Virginia University in partial fulfillment of Requirements -
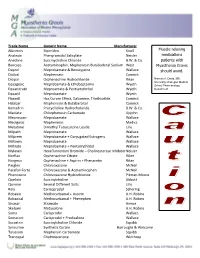
Medication Alert
Trade Name Generic Name Manufacturer Akineton Biperiden Knoll Muscle relaxing Analexin Phenyramidol Salicylate Neisler medications Anectine Succinycholine Chloride B.W. & Co. patients with Bancaps Acetaminophin, Mephenesin Butabarbital Sodium West Myasthenia Gravis Deprol Meprobamate & Benscyyzine Wallace should avoid. Dioloxl Mephensein Camrick Disipal Orphenadrine Hydrochloride Riker Norman A. David, MD, University of Oregon Medical Equagesic Meprobamate & Ethobeptazine Wyeth School Pharmacology Equanitrate Meproamate & Pentserythritol Wyeth Department Equanil Meprobamate Wyeth Flaxedil Has Curare Effect, Galiamine, Triethiodide Camrick Halabar Mephenesin & Butabarbital Camrick Kemadrin Procycllidine Hydrocholoride B.W. & Co. Maolate Chlorphenesin Carbamate Upjohn Meprospan Meprobamate Wallace Medigesic Mephenesin Medics Metubine Dimethyl Tubocurzine Loside Lilly Milpath Meprobamate Wallace Milprem Meprobamate + Conjugated Estrogens Wallace Miltown Meprobamate Wallace Miltrate Meprobamate + Pentserythritol Wallace Mylaxen Hexafluroenium Bromide – Cholinesterase Inhibitor Neisler Norflax Orphenadrine Citrate Riker Norgesic Orphenadrine + Aspirin + Phenacetin Riker Parglex Chlorzoxazone McNeil Parafon Forte Chlorzoxazone & Acetaminophen McNeil Phenoxene Chlorzoxazone Hydrochloride Pitman-Moore Quelicin Succinylcholine Abbott Quinine Several Different Salts Lilly Rela Carisoprodol Schering Robaxin Methocarbamal + Aspirin A.H. Robins Robaxisal Methocarbamal + Phenephen A.H. Robins Sinaxar Stramate Armor Skelaxin Metaxalone A.H. Robins Soma