Hydrogen Bonding in Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Clusters in Plants. Fast Channel Plant Communications. Planet Influence
Vol.1, No.1, 1-11 (2010) Journal of Biophysical Chemistry doi:10.4236/jbpc.2010.11001 Water clusters in plants. Fast channel plant communications. Planet influence Kristina Zubow1, Anatolij Viktorovich Zubow2, Viktor Anatolijevich Zubow1* 1“Aist Handels- und consulting GmbH”, R&D Department, Groß Gievitz, Germany; *Corresponding Author: [email protected] 2Department of Computer Science, Humboldt University Berlin, Berlin, Germany; [email protected] Received 19 April, 2010; revised 30 April, 2010; accepted 7 May 2010. ABSTRACT 1. INTRODUCTION In tubers of two potato cultivars and in one ap- It is well known, that in the bulk water molecules form ple cultivar, water clusters, consisting of 11 ± 1, clusters [1]. Though clusters were discovered in bio- 100, 178, 280, 402, 545, 715, 903, 119, 1351, 1606 logical matrices [2] until now there isn’t still a method and 1889 molecules, were directly (in-vivo) by which clusters in plants can be identified during analyzed by gravitation spectroscopy. The growth in-vivo. By Okonchi Shoichi [3] it was suggested, clusters’ interactions with their surroundings that water molecules are in the form of clusters in living during plant growth in summer 2006 in Germany organisms. In Figure 1 computer models of some water were described where a model represents the clusters are given. In our laboratory, we developed a states of water clusters in bio matrices. Fur- gravitation spectrometer for water cluster identification thermore, a comparison with clusters in irriga- in bio-matrices of plants [4-6]. Knowing the state of tion water (river, rain) is given. To achieve a high water clusters in plants could be helpful for understand- and good quality yield it is necessary to choose ing the relation between biochemical processes at nano- the right irrigation water that has to correspond scale level during growth and qualitative yields. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Water Cluster Ensembles As Interface to the Structure of Epicenter and the Earthquake Mechanism on the Jawa Island ´ S
id4277265 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com ISSN : 0974 - 7524 Volume 7 Issue 3 Physical CCHHEEAMMn InIIdSSiaTnT JRoRurYnYal Trade Science Inc. Full Paper PCAIJ, 7(3), 2012 [87-95] Water cluster ensembles as interface to the structure of epicenter and the earthquake mechanism on the Jawa island ´ s. 112 ´ e.) (8 73 36 K.Zubow2, A.Zubow1, V.A.Zubow2* 1Dept.of Computer Science, Humboldt University Berlin, Johann von Neumann Haus, House III, 3rd Floor, Rudower Chaussee 25, D-12489 (BERLIN) 2«A IST H&C», Dept.R&D, D-17192 Groß Gievitz, Dorfstraße, 3, (GERMANY) E-mail: [email protected]; [email protected] Received: 6th December, 2011 ; Accepted: 7th January, 2012 ABSTRACT KEYWORDS The long-range order (LRO) in underground water (North Germany) that Water; was excited by gravitation was investigated in the period of August 13th to Clusters; 14th, 2009. The average molecular mass of water clusters, the forms of the Epicenter; Structure; base water cluster (H2O)12 and of the Chaplin cluster (H2O)280, were found to correlate with a series of gravitation excitation which is connected with Earthquake; the earthquake on the Java island. It has been investigated how earth- Mechanism; quakes at distance influence the general energy capacity of the water Jawa. cluster ensembles. A mechanism describing generation and development of gravitation tensions in the epicenter on Java as the result of changed hydrogen bridges in water under the influence of impulse pressure up to 0.46 GPa has been suggested. -

BEYOND the STATUS QUO: 2015 EQB Water Policy Report
BEYOND THE STATUS QUO: 2015 EQB Water Policy Report LAKE ST. CROIX TABLE OF CONTENTS Introduction . 4 Health Equity and Water. 5 GOAL #1: Manage Water Resources to Meet Increasing Demands . .6 GOAL #2: Manage Our Built Environment to Protect Water . 14 GOAL #3: Increase and Maintain Living Cover Across Watersheds .. 20 GOAL #4: Ensure We Are Resilient to Extreme Rainfall . .28 Legislative Charge The Environmental Quality Board is mandated to produce a five year water Contaminants of Emerging Concern . .34 policy report pursuant to Minnesota Statutes, sections 103A .204 and 103A .43 . Minnesota’s Water Technology Industry . 36 This report was prepared by the Environmental Quality Board with the Board More Information . .43 of Water and Soil Resources, Department of Agriculture, Department of Employment and Economic Development, Department of Health, Department Appendices available online: of Natural Resources, Department of Transportation, Metropolitan Council, • 2015 Groundwater Monitoring Status Report and Pollution Control Agency . • Five-Year Assessment of Water Quality Degradation Trends and Prevention Efforts Edited by Mary Hoff • Minnesota’s Water Industry Economic Profile Graphic Design by Paula Bohte • The Agricultural BMP Handbook for Minnesota The total cost of preparing this report was $76,000 • Water Availability Assessment Report 2 Beyond the Status Quo: 2015 EQB Water Policy Report Minnesota is home to more than 10,000 lakes, 100,000 miles of rivers and streams, and abundant groundwater resources. However, many of these waters are not clean enough. In 2015, we took a major step toward improving our water by enacting a law that protects water quality by requiring buffers on more than 100,000 acres of land adjacent to water. -

Power Sources Challenge
POWER SOURCES CHALLENGE FUSION PHYSICS! A CLEAN ENERGY Summary: What if we could harness the power of the Sun for energy here on Fusion reactions occur when two nuclei come together to form one Earth? What would it take to accomplish this feat? Is it possible? atom. The reaction that happens in the sun fuses two Hydrogen atoms together to produce Helium. It looks like this in a very simplified way: Many researchers including our Department of Energy scientists and H + H He + ENERGY. This energy can be calculated by the famous engineers are taking on this challenge! In fact, there is one DOE Laboratory Einstein equation, E = mc2. devoted to fusion physics and is committed to being at the forefront of the science of magnetic fusion energy. Each of the colliding hydrogen atoms is a different isotope of In order to understand a little more about fusion energy, you will learn about hydrogen, one deuterium and one the atom and how reactions at the atomic level produce energy. tritium. The difference in these isotopes is simply one neutron. Background: It all starts with plasma! If you need to learn more about plasma Deuterium has one proton and one physics, visit the Power Sources Challenge plasma activities. neutron, tritium has one proton and two neutrons. Look at the The Fusion Reaction that happens in the SUN looks like this: illustration—do you see how the mass of the products is less than the mass of the reactants? That is called a mass deficit and that difference in mass is converted into energy. -

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

2.#Water;#Acid.Base#Reac1ons
8/24/15 BIOCH 755: Biochemistry I Fall 2015 2.#Water;#Acid.base#reac1ons# Jianhan#Chen# Office#Hour:#M#1:30.2:30PM,#Chalmers#034# Email:#[email protected]# Office:#785.2518# 2.1#Physical#Proper1es#of#Water# • Key$Concepts$2.1$ – Water#molecules,#which#are#polar,#can#form#hydrogen#bonds#with# other#molecules.# – In#ice,#water#molecules#are#hydrogen#bonded#in#a#crystalline#array,#but# in#liquid#water,#hydrogen#bonds#rapidly#break#and#re.form#in#irregular# networks.# – The#aTrac1ve#forces#ac1ng#on#biological#molecules#include#ionic# interac1ons,#hydrogen#bonds,#and#van#der#Waals#interac1ons.# – Polar#and#ionic#substances#can#dissolve#in#water.# (c)#Jianhan#Chen# 2# 1 8/24/15 2.1#Physical#Proper1es#of#Water# • Key$Concepts$2.1$ – The#hydrophobic#effect#explains#the#exclusion#of#nonpolar#groups#as#a# way#to#maximize#the#entropy#of#water#molecules.# – Amphiphilic#substances#form#micelles#or#bilayers#that#hide#their# hydrophobic#groups#while#exposing#their#hydrophilic#groups#to#water.# – Molecules#diffuse#across#membranes#which#are#permeable#to#them# from#regions#of#higher#concentra1on#to#regions#of#lower# concentra1on.# – In#dialysis,#solutes#diffuse#across#a#semipermeable#membrane#from# regions#of#higher#concentra1on#to#regions#of#lower#concentra1on.# (c)#Jianhan#Chen# 3# Human#Body#Mass#Composi1on# (c)#Jianhan#Chen# 4 2 8/24/15 Structure#of#Water# (c)#Jianhan#Chen# 5 Water#Hydrogen#Bonding# ~1.8 Å, 180o Acceptor Donor (c)#Jianhan#Chen# 6# 3 8/24/15 Typical#Bond#Energies# (c)#Jianhan#Chen# 7# Hydrogen#bond#networks#of#water/ice# (c)#Jianhan#Chen# -

Unique Properties of Water!
Name: _______ANSWER KEY_______________ Class: _____ Date: _______________ Unique Properties of Water! Word Bank: Adhesion Evaporation Polar Surface tension Cohesion Freezing Positive Universal solvent Condensation Melting Sublimation Dissolve Negative 1. The electrons are not shared equally between the hydrogen and oxygen atoms of water creating a Polar molecule. 2. The polarity of water allows it to dissolve most substances. Because of this it is referred to as the universal solvent 3. Water molecules stick to other water molecules. This property is called cohesion. 4. Hydrogen bonds form between adjacent water molecules because the positive charged hydrogen end of one water molecule attracts the negative charged oxygen end of another water molecule. 5. Water molecules stick to other materials due to its polar nature. This property is called adhesion. 6. Hydrogen bonds hold water molecules closely together which causes water to have high surface tension. This is why water tends to clump together to form drops rather than spread out into a thin film. 7. Condensation is when water changes from a gas to a liquid. 8. Sublimation is when water changes from a solid directly to a gas. 9. Freezing is when water changes from a liquid to a solid. 10. Melting is when water changes from a solid to a liquid. 11. Evaporation is when water changes from a liquid to a gas. 12. Why does ice float? Water expands as it freezes, so it is LESS DENSE AS A SOLID. 13. What property refers to water molecules resembling magnets? How are these alike? Polar bonds create positive and negative ends of the molecule. -
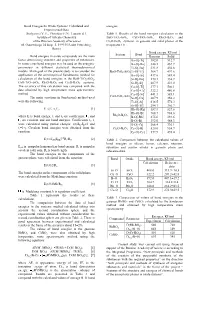
Bond Energies in Oxide Systems: Calculated and Experimental Data
Bond Energies In Oxide Systems: Calculated and energies. Experimental Data Stolyarova V. L., Plotnikov E.N., Lopatin S.I. Table 1. Results of the bond energies calculation in the Institute of Silicate Chemistry BaO-TiO 2-SiO 2, CaO-TiO 2-SiO 2, Rb 2O-B2O3 and of the Russian Academy of Sciences Cs 2O-B2O3 systems. in gaseous and solid phases at the ul. Odoevskogo 24 korp. 2, 199155 Sankt Petersburg, temperature 0 ¥ . Russia Bond energy, KJ/mol System Bond Bond energies in oxide compounds are the main Gaseous Solid factor determining structure and properties of substances. Ba-O[-Ti] 382.0 512.7 In some cases bond energies may be used as the energetic Ba-O[-Ba] 348.5 467.7 parameters in different statistical thermodynamical Ti-O[-Ba] 331.8 450.6 models. Main goal of the present study is to consider the BaO-TiO 2-SiO 2 Ti-O[-Ti] 364.7 497.6 application of the semiempirical Sanderson's method for Ba-O[-Si] 437.6 585.4 calculation of the bond energies in the BaO-TiO 2-SiO 2, Si-O[-Ba] 270.3 334.7 CaO-TiO 2-SiO 2, Rb 2O-B2O3 and Cs 2O-B2O3 systems. Si-O[-Si] 407.9 421.0 The accuracy of this calculation was compared with the Ca-O[-Ti] 377.1 500.1 data obtained by high temperature mass spectrometric Ca-O[-Ca] 322.2 486.8 method. Ca-O[-Si] 441.1 585.1 CaO-TiO -SiO The main equations in Sanderson's method used 2 2 Si-O[-Ca] 287.9 358.2 were the following. -

Molecular Orbital Theory to Predict Bond Order • to Apply Molecular Orbital Theory to the Diatomic Homonuclear Molecule from the Elements in the Second Period
Skills to Develop • To use molecular orbital theory to predict bond order • To apply Molecular Orbital Theory to the diatomic homonuclear molecule from the elements in the second period. None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals, each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals (AOs), the positions and energies of electrons in molecules can be described in terms of molecular orbitals (MOs) A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy.—a spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. -

Lithium Hydride Powered PEM Fuel Cells for Long-Duration Small Mobile Robotic Missions
Lithium Hydride Powered PEM Fuel Cells for Long-Duration Small Mobile Robotic Missions Jekanthan Thangavelautham, Daniel Strawser, Mei Yi Cheung Steven Dubowsky Abstract— This paper reports on a study to develop power supplies for small mobile robots performing long duration missions. It investigates the use of fuel cells to achieve this objective, and in particular Proton Exchange Membrane (PEM) fuel cells. It is shown through a representative case study that, in theory, fuel cell based power supplies will provide much longer range than the best current rechargeable battery technology. It also briefly discusses an important limitation that prevents fuel cells from achieving their ideal performance, namely a practical method to store their fuel (hydrogen) in a form that is compatible with small mobile field robots. A very efficient Fig. 1. Example of small robots (Left) A ball shaped hopping robot concept fuel storage concept based on water activated lithium hydride for exploration of extreme terrains and caves developed for NASA. (Right) (LiH) is proposed that releases hydrogen on demand. This iRobot 110 Firstlook used for observation, security and search and rescue. concept is very attractive because water vapor from the air is passively extracted or waste water from the fuel cell is recycled and transferred to the lithium hydride where the hydrogen is the near future. Hence, new means for powering field robots “stripped” from water and is returned to the fuel cell to form more water. This results in higher hydrogen storage efficiencies need to be considered. than conventional storage methods. Experimental results are This paper explores the use of fuel cells, and in particular presented that demonstrate the effectiveness of the approach. -

A New Way for Probing Bond Strength J
A New Way for Probing Bond Strength J. Klein, H. Khartabil, J.C. Boisson, J. Contreras-Garcia, Jean-Philip Piquemal, E. Henon To cite this version: J. Klein, H. Khartabil, J.C. Boisson, J. Contreras-Garcia, Jean-Philip Piquemal, et al.. A New Way for Probing Bond Strength. Journal of Physical Chemistry A, American Chemical Society, 2020, 124 (9), pp.1850-1860. 10.1021/acs.jpca.9b09845. hal-02377737 HAL Id: hal-02377737 https://hal.archives-ouvertes.fr/hal-02377737 Submitted on 27 Mar 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A New Way for Probing Bond Strength Johanna Klein,y Hassan Khartabil,y Jean-Charles Boisson,z Julia Contreras-Garc´ıa,{ Jean-Philip Piquemal,{ and Eric H´enon∗,y yInstitut de Chimie Mol´eculaire de Reims UMR CNRS 7312, Universit´ede Reims Champagne-Ardenne, Moulin de la Housse 51687 Reims Cedex 02 BP39 (France) zCReSTIC EA 3804, Universit´ede Reims Champagne-Ardenne, Moulin de la Housse 51687 Reims Cedex 02 BP39 (France) {Sorbonne Universit´es,UPMC, Laboratoire de Chimie Th´eoriqueand UMR CNRS 7616, 4 Pl Jussieu, 75252 Paris Cedex 05(France) E-mail: [email protected] Phone: +33(3)26918497 1 Abstract The covalent chemical bond is intimately linked to electron sharing between atoms.