Expression of Smooth Muscle and Extracellular Matrix Proteins in Relation to Airway Function in Asthma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary File 2A Revised
Supplementary file 2A. Differentially expressed genes in aldosteronomas compared to all other samples, ranked according to statistical significance. Missing values were not allowed in aldosteronomas, but to a maximum of five in the other samples. Acc UGCluster Name Symbol log Fold Change P - Value Adj. P-Value B R99527 Hs.8162 Hypothetical protein MGC39372 MGC39372 2,17 6,3E-09 5,1E-05 10,2 AA398335 Hs.10414 Kelch domain containing 8A KLHDC8A 2,26 1,2E-08 5,1E-05 9,56 AA441933 Hs.519075 Leiomodin 1 (smooth muscle) LMOD1 2,33 1,3E-08 5,1E-05 9,54 AA630120 Hs.78781 Vascular endothelial growth factor B VEGFB 1,24 1,1E-07 2,9E-04 7,59 R07846 Data not found 3,71 1,2E-07 2,9E-04 7,49 W92795 Hs.434386 Hypothetical protein LOC201229 LOC201229 1,55 2,0E-07 4,0E-04 7,03 AA454564 Hs.323396 Family with sequence similarity 54, member B FAM54B 1,25 3,0E-07 5,2E-04 6,65 AA775249 Hs.513633 G protein-coupled receptor 56 GPR56 -1,63 4,3E-07 6,4E-04 6,33 AA012822 Hs.713814 Oxysterol bining protein OSBP 1,35 5,3E-07 7,1E-04 6,14 R45592 Hs.655271 Regulating synaptic membrane exocytosis 2 RIMS2 2,51 5,9E-07 7,1E-04 6,04 AA282936 Hs.240 M-phase phosphoprotein 1 MPHOSPH -1,40 8,1E-07 8,9E-04 5,74 N34945 Hs.234898 Acetyl-Coenzyme A carboxylase beta ACACB 0,87 9,7E-07 9,8E-04 5,58 R07322 Hs.464137 Acyl-Coenzyme A oxidase 1, palmitoyl ACOX1 0,82 1,3E-06 1,2E-03 5,35 R77144 Hs.488835 Transmembrane protein 120A TMEM120A 1,55 1,7E-06 1,4E-03 5,07 H68542 Hs.420009 Transcribed locus 1,07 1,7E-06 1,4E-03 5,06 AA410184 Hs.696454 PBX/knotted 1 homeobox 2 PKNOX2 1,78 2,0E-06 -

Supplemental Figure Legends Figure S1. Expression of Mir-133A
Supplemental Figure legends Figure S1. Expression of miR-133a and miR-1 in adult mice. (A) Expression of miR-133a in heart samples of miR-133a-1-KO and miR-133a-2-KO adult mice as detected by real-time PCR. Expression level of miR-133a in mutant hearts were normalized to GAPDH and compared to WT hearts. N=3 for each genotype group. (B) Northern blot analysis of heart and skeletal muscle RNA from adult mice. Ten micrograms of RNA from skeletal muscle and heart tissues were used in the Northern blots. 32P-labeled Start-Fire probes for miR-133a and miR-1 were used. Genotypes of mice are labeled on top. U6 probe was used as a loading control. GP, gastrocnemius- plantaris. (C) Expression of Mib1 mRNA and protein in dKO mice at P1. mRNA level of Mib1 as detected by real-time PCR in dKO hearts were normalized to GAPDH and compared to WT hearts. Error bars indicate SEM. Western blot analysis was performed on hearts from P1 wild type and dKO mutant mice. α -tubulin was detected as a loading control. Figure S2. Abnormal cardiomyocyte proliferation in miR-133a dKO hearts. Representative images of proliferating cardiomyocytes in WT and dKO hearts at P1. High magnification of immunohistochemistry on heart sections at P1 using phospho- histone H3 (PH3, red) and -actinin (green) was shown. Bar = 20 m. Figure S3. Expression of miR-133a in wild-type and beta-MHC-miR-133a transgenic hearts at E13.5 as detected by real-time PCR. Figure S4. Expression of miR-133a target genes in hearts of WT and dKO mutant mice at P1. -

Snapshot: Actin Regulators II Anosha D
SnapShot: Actin Regulators II Anosha D. Siripala and Matthew D. Welch Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720, USA Representative Proteins Protein Family H. sapiens D. melanogaster C. elegans A. thaliana S. cerevisiae Endocytosis and Exocytosis ABP1/drebrin mABP1, drebrin, drebrin- †Q95RN0 †Q9XUT0 Abp1 like EPS15 EPS15 Eps-15 EHS-1 †Q56WL2 Pan1 HIP1R HIP1R †Q8MQK1 †O62142 Sla2 Synapsin synapsin Ia, Ib, IIa, IIb, III Synapsin SNN-1 Plasma Membrane Association Anillin anillin Scraps ANI-1, 2, 3 Annexins annexin A1–11, 13 (actin Annexin B9-11 NEX-1–4 ANN1-8 binding: 1, 2, 6) ERM proteins ezrin, radixin, moesin DMoesin ERM-1 MARCKS MARCKS, MRP/ Akap200 MACMARCKS/F52 Merlin *merlin/NF2 Merlin NFM-1 Protein 4.1 4.1R, G, N, B Coracle Spectrin α-spectrin (1–2), β-spectrin α-spectrin, β-spectrin, β heavy- SPC-1 (α-spectrin), UNC-70 (1–4), β heavy-spectrin/ spectrin/Karst (β-spectrin), SMA-1 (β heavy- karst spectrin) Identifi ed Cellular Role: X Membrane traffi cking and phagocytosis Cell-Cell Junctions X Cytokinesis α-catenin α-catenin 1–3 α-catenin HMP-1 X Cell surface organization and dynamics X Cell adhesion Afadin afadin/AF6 Canoe AFD-1 X Multiple functions ZO-1 ZO-1, ZO-2, ZO-3 ZO-1/Polychaetoid †Q56VX4 X Other/unknown Cell-Extracellular Matrix Junctions †UNIPROT database accession number *Mutation linked to human disease Dystrophin/utrophin *dystrophin, utrophin/ Dystrophin DYS-1 DRP1, DRP2 LASP LASP-1, LASP-2, LIM- Lasp †P34416 nebulette Palladin palladin Parvin α-, β-, χ-parvin †Q9VWD0 PAT-6 -

Potential Differentiation of Human Mesenchymal Stem Cell Transplanted in Rat Corpus Cavernosum Toward Endothelial Or Smooth Muscle Cells
International Journal of Impotence Research (2007) 19, 378–385 & 2007 Nature Publishing Group All rights reserved 0955-9930/07 $30.00 www.nature.com/ijir ORIGINAL ARTICLE Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells YS Song1,6,HJLee2,6,IHPark2, WK Kim3,JHKu4, SU Kim3,5 1Department of Urology, Soonchunhyang School of Medicine, Seoul, Korea; 2Brain Disease Research Center, Ajou University School of Medicine, Suwon, Korea; 3Institute of Regeneration Medicine, Gacheon University Ghill Hospital, Inchon, Korea; 4Department of Urology, Seoul National University Hospital, Seoul, Korea and 5Division of Neurology, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada One of the causes of erectile dysfunction (ED) is the damaged penile cavernous smooth muscle cells (SMCs) and sinus endothelial cells (ECs). To investigate the feasibility of applying immortalized human mesenchymal stem cells (MSCs) to penile cavernous ECs or SMCs repair in the treatment of ED, the in vivo potential differentiation of the immortalized human MSCs toward penile cavernous endothelial or smooth muscle was investigated. One clone of immortalized human bone marrow mesenchymal stem cell line B10 cells via retroviral vector encoding v-myc were transplanted into the cavernosum of the Sprague–Dawley rats and harvested 2 weeks later. The expression of CD31, von Willebrand factor (vWF), smooth muscle cell actin (SMA), calponin and desmin was determined immunohistochemically in rat penile cavernosum. Multipotency of B10 to adipogenic, osteogenic or chondrogenic differentiation was found. Expression of EC specific markers (CD31 or vWF protein) and expression of SMC specific markers (calponin, SMA or desmin protein) were demonstrated in grafted B10 cells. -

Calponin 1 CRISPR/Cas9 KO Plasmid (M): Sc-419719
SANTA CRUZ BIOTECHNOLOGY, INC. Calponin 1 CRISPR/Cas9 KO Plasmid (m): sc-419719 BACKGROUND APPLICATIONS The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and Calponin 1 CRISPR/Cas9 KO Plasmid (m) is recommended for the disruption CRISPR-associated protein (Cas9) system is an adaptive immune response of gene expression in mouse cells. defense mechanism used by archea and bacteria for the degradation of foreign genetic material (4,6). This mechanism can be repurposed for other 20 nt non-coding RNA sequence: guides Cas9 functions, including genomic engineering for mammalian systems, such as to a specific target location in the genomic DNA gene knockout (KO) (1,2,3,5). CRISPR/Cas9 KO Plasmid products enable the U6 promoter: drives gRNA scaffold: helps Cas9 identification and cleavage of specific genes by utilizing guide RNA (gRNA) expression of gRNA bind to target DNA sequences derived from the Genome-scale CRISPR Knock-Out (GeCKO) v2 library developed in the Zhang Laboratory at the Broad Institute (3,5). Termination signal Green Fluorescent Protein: to visually REFERENCES verify transfection CRISPR/Cas9 Knockout Plasmid CBh (chicken β-Actin 1. Cong, L., et al. 2013. Multiplex genome engineering using CRISPR/Cas hybrid) promoter: drives systems. Science 339: 819-823. 2A peptide: expression of Cas9 allows production of both Cas9 and GFP from the 2. Mali, P., et al. 2013. RNA-guided human genome engineering via Cas9. same CBh promoter Science 339: 823-826. Nuclear localization signal 3. Ran, F.A., et al. 2013. Genome engineering using the CRISPR-Cas9 system. Nuclear localization signal SpCas9 ribonuclease Nat. Protoc. 8: 2281-2308. -

Inhibition of Human Prostate Smooth Muscle Contraction by the LIM Kinase Inhibitors, SR7826 and Limki3
Aus der Urologischen Klinik und Poliklinik der Ludwig-Maximilians-Universität München Direktor: Prof. Dr. med. Christian G. Stief Inhibition of human prostate smooth muscle contraction by the LIM kinase inhibitors, SR7826 and LIMKi3 Dissertation zum Erwerb des Doktorgrades der Medizin an der Medizinischen Fakultät der Ludwig-Maximilians-Universität zu München vorgelegt von Qingfeng Yu aus Guangdong, China 2018 Mit Genehmigung der Medizinischen Fakultät der Universität München Berichterstatter: Prof. Dr. med. Christian. G. Stief Mitberichterstatter: Priv. Dr. Claudia Veigel Priv. Dr. Oliver Reich Mitbetreuung durch den Promovierten Mitarbeiter: Priv. Doz. Dr. rer. nat. Martin Hennenberg Dekan: Prof. Dr. med. dent. Reinhard Hickel Tag der mündlichen Prüfung: 19.04.2018 CONTENTS Contents 1 Introduction ............................................................................................................... 1 1.1 Definition ........................................................................................................ 1 1.2 Epidemiology .................................................................................................. 1 1.3 Etiology ........................................................................................................... 2 1.4 The role of BPH in LUTS ............................................................................... 5 1.5 Pharmacological treatment for LUTS .............................................................. 7 1.5.1 Current therapies ................................................................................. -

Are You Suprised ?
RESEARCH USE ONLY DATA SHEET Rev 040516F Clinical customers please refer to IVD / ASR Data Sheet Calponin-1 (Clone EP798Y) Rabbit Monoclonal Antibody Produced by Epitomics, Inc. Using Technology Licensed Under Patent no. 5,675,063 Cat. #RM-2102-S0, or -S (0.1ml or 1.0ml Supernatant) (Purified Ab with BSA and Azide) Cat. #RM-2102-R7 (7.0ml) (Ready-to-Use for Immunohistochemistry) Cat. #RM-2102-RQ (12.0ml) (Ready-to-Use for Immunohistochemistry) Please note this data sheet has been changed effective April 5, 2016 Description: Calponin, a thin filament associated protein Storage and Stability: is implicated in the regulation and modulation of smooth Store vial at 40C. When stored at 2-80C, this antibody is muscle contraction. It is capable of binding to actin, stable for 24 months. calmodulin, troponin C and tropomyosin. Calponin is expressed in smooth muscle and tissues containing Suggested References: significant amounts of smooth muscle. Two isoforms of 1. Douglas-Jones A et al. Histopathology 2005;47:202 calponin exist whose molecular weights are 34kDa and 2. Rabban J T et al. Mod Path 2006;19:1351 29kDa. Expression of the 29kDa form is primarily restricted 3. Werling R W et al. AJSP 2003;27:82, to muscle of the urogenital tract. The expression of 4. Lazard D et al. Proc Natl Acad Sci USA 1993;90:999 calponin has also been demonstrated in myoepithelial cells 5. Zhang R R et al. Breast Cancer Res 2003;5:R151-6 from benign and malignant breast lesions. It stains smooth muscle, myoepithelial cells, myofibroblasts, keratinocytes Limitations and Warranty: and nerve fibers. -

Actin Stress Fibers – Assembly, Dynamics and Biological Roles
Commentary 1855 Actin stress fibers – assembly, dynamics and biological roles Sari Tojkander, Gergana Gateva and Pekka Lappalainen* Institute of Biotechnology, P.O. Box 56, University of Helsinki, 00014, Finland *Author for correspondence ([email protected]) Journal of Cell Science 125, 1855–1864 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.098087 Summary Actin filaments assemble into diverse protrusive and contractile structures to provide force for a number of vital cellular processes. Stress fibers are contractile actomyosin bundles found in many cultured non-muscle cells, where they have a central role in cell adhesion and morphogenesis. Focal-adhesion-anchored stress fibers also have an important role in mechanotransduction. In animal tissues, stress fibers are especially abundant in endothelial cells, myofibroblasts and epithelial cells. Importantly, recent live-cell imaging studies have provided new information regarding the mechanisms of stress fiber assembly and how their contractility is regulated in cells. In addition, these studies might elucidate the general mechanisms by which contractile actomyosin arrays, including muscle cell myofibrils and cytokinetic contractile ring, can be generated in cells. In this Commentary, we discuss recent findings concerning the physiological roles of stress fibers and the mechanism by which these structures are generated in cells. Key words: Actin, Adhesion, Assembly, Mechanotransduction, Stress fibers Introduction muscle cells and stress fibers of non-muscle -

Calponin-3 Deficiency Augments Contractile Activity, Plasticity
www.nature.com/scientificreports OPEN Calponin-3 defciency augments contractile activity, plasticity, fbrogenic response and Yap/Taz transcriptional activation in lens epithelial cells and explants Rupalatha Maddala1, Maureen Mongan2, Ying Xia2 & Ponugoti Vasantha Rao1,3* The transparent ocular lens plays a crucial role in vision by focusing light on to the retina with loss of lens transparency leading to impairment of vision. While maintenance of epithelial phenotype is recognized to be essential for lens development and function, knowledge of the identity of diferent molecular mechanisms regulating lens epithelial characteristics remains incomplete. This study reports that CNN-3, the acidic isoform of calponin, an actin binding contractile protein, is expressed preferentially and abundantly relative to the basic and neutral isoforms of calponin in the ocular lens, and distributes predominantly to the epithelium in both mouse and human lenses. Expression and MEKK1-mediated threonine 288 phosphorylation of CNN-3 is induced by extracellular cues including TGF-β2 and lysophosphatidic acid. Importantly, siRNA-induced defciency of CNN3 in lens epithelial cell cultures and explants results in actin stress fber reorganization, stimulation of focal adhesion formation, Yap activation, increases in the levels of α-smooth muscle actin, connective tissue growth factor and fbronectin, and decreases in E-cadherin expression. These results reveal that CNN3 plays a crucial role in regulating lens epithelial contractile activity and provide supporting evidence that CNN-3 defciency is associated with the induction of epithelial plasticity, fbrogenic activity and mechanosensitive Yap/Taz transcriptional activation. Te avascular transparent ocular lens plays a crucial role in vision by focusing incident light on to the retina and aberrations in lens transparency (cataract) impair vision. -
Supplementary Figures S1-S3
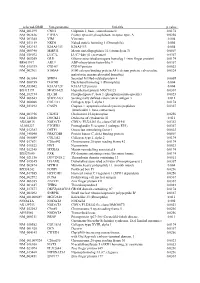
selected-GBID Uni-genename Uni-title p value NM_001299 CNN1 Calponin 1, basic, smooth muscle 0.0174 NM_002836 PTPRA Protein tyrosine phosphatase, receptor type, A 0.0256 NM_003380 VIM Vimentin 0.004 NM_033119 NKD1 Naked cuticle homolog 1 (Drosophila) 0.004 NM_052913 KIAA1913 KIAA1913 0.004 NM_005940 MMP11 Matrix metallopeptidase 11 (stromelysin 3) 0.0069 NM_018032 LUC7L LUC7-like (S. cerevisiae) 0.0367 NM_005269 GLI1 Glioma-associated oncogene homolog 1 (zinc finger protein) 0.0174 BE463997 ARL9 ADP-ribosylation factor-like 9 0.0367 NM_015939 CGI-09 CGI-09 protein 0.0023 NM_002961 S100A4 S100 calcium binding protein A4 (calcium protein, calvasculin, 0.0324 metastasin, murine placental homolog) NM_003014 SFRP4 Secreted frizzled-related protein 4 0.0005 NM_080759 DACH1 Dachshund homolog 1 (Drosophila) 0.004 NM_053042 KIAA1729 KIAA1729 protein 0.004 BX415194 MGC16121 Hypothetical protein MGC16121 0.0367 NM_182734 PLCB1 Phospholipase C, beta 1 (phosphoinositide-specific) 0.0023 NM_006643 SDCCAG3 Serologically defined colon cancer antigen 3 0.011 NM_000088 COL1A1 Collagen, type I, alpha 1 0.0174 NM_033292 CASP1 Caspase 1, apoptosis-related cysteine peptidase 0.0367 (interleukin 1, beta, convertase) NM_003956 CH25H Cholesterol 25-hydroxylase 0.0256 NM_144658 DOCK11 Dedicator of cytokinesis 11 0.011 AK024935 NODATA CDNA: FLJ21283 fis, clone COL01910 0.0363 AL050227 PTGER3 Prostaglandin E receptor 3 (subtype EP3) 0.0367 NM_012383 OSTF1 Osteoclast stimulating factor 1 0.0023 NM_145040 PRKCDBP Protein kinase C, delta binding protein 0.0069 NM_000089 -

Calponin-1 (Clone EP798Y) Rabbit Monoclonal Antibody Produced by Epitomics, Inc
RESEARCH USE ONLY DATA SHEET Rev 121411D Clinical customers please refer to IVD / ASR Data Sheet Calponin-1 (Clone EP798Y) Rabbit Monoclonal Antibody Produced by Epitomics, Inc. Using Technology Licensed Under Patent no. 5,675,063 Cat. #RM-2102-S0, or -S (0.1ml or 1.0ml Supernatant) (Purified Ab with BSA and Azide) Cat. #RM-2102-R7 (7.0ml) (Ready-to-Use for Immunohistochemistry) Cat. #RM-2102-RQ (12.0ml) (Ready-to-Use for Immunohistochemistry) Please note this data sheet has been changed effective December 14, 2011 Description: Calponin, a thin filament associated protein Storage and Stability: is implicated in the regulation and modulation of smooth Store vial at 40C. When stored at 2-80C, this antibody is muscle contraction. It is capable of binding to actin, stable for 24 months. calmodulin, troponin C and tropomyosin. Calponin is expressed in smooth muscle and tissues containing Suggested References: significant amounts of smooth muscle. Two isoforms of 1. Douglas-Jones A et al. Histopathology 2005;47:202 calponin exist whose molecular weights are 34kDa and 2. Rabban J T et al. Mod Path 2006;19:1351 29kDa. Expression of the 29kDa form is primarily restricted 3. Werling R W et al. AJSP 2003;27:82, to muscle of the urogenital tract. The expression of 4. Lazard D et al. Proc Natl Acad Sci USA 1993;90:999 calponin has also been demonstrated in myoepithelial cells 5. Zhang R R et al. Breast Cancer Res 2003;5:R151-6 from benign and malignant breast lesions. It stains smooth muscle, myoepithelial cells, myofibroblasts, keratinocytes Limitations and Warranty: and nerve fibers. -

Human Calponin 1 Antibody
Human Calponin 1 Antibody Monoclonal Mouse IgG3 Clone # 836701 Catalog Number: MAB7900 DESCRIPTION Species Reactivity Human Specificity Detects human Calponin 1 in ELISAs. Detects human, mouse and rat Calponin 1 in Western Blots. In direct ELISAs, no crossreactivity with recombinant human Calponin 3 is observed. Source Monoclonal Mouse IgG3 Clone # 836701 Purification Protein A or G purified from hybridoma culture supernatant Immunogen E. coliderived recombinant human Calponin 1 Ser2Ala297 Accession # P51911 Formulation Lyophilized from a 0.2 μm filtered solution in TBS with Trehalose. See Certificate of Analysis for details. *Small pack size (SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. APPLICATIONS Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website. Recommended Sample Concentration Western Blot 1 µg/mL See Below Immunocytochemistry 825 µg/mL See Below Immunohistochemistry 825 µg/mL See Below DATA Western Blot Immunocytochemistry Detection of Human, Mouse, and Calponin 1 in MDAMD231 Human Cell Rat Calponin 1 by Western Blot. Western Line. Calponin 1 was detected in immersion blot shows lysates of MDAMB231 human fixed MDAMD231 human breast cancer cell breast cancer cell line, MCF7 human breast line using Mouse AntiHuman Calponin 1 cancer cell line, NMuMG mouse mammary Monoclonal Antibody (Catalog # MAB7900) gland epithelial cell line, and A7r5 rat at 10 µg/mL for 3 hours at room temperature. thoracic aortic smooth muscle cell line. Cells were stained using the Northern PVDF membrane was probed with 1 µg/mL of Lights™ 557conjugated AntiMouse IgG Mouse AntiHuman Calponin 1 Monoclonal Secondary Antibody (red; Catalog # NL007) Antibody (Catalog # MAB7900) followed by and counterstained with DAPI (blue).