Effect of Low Temperature on Changes in AGP Distribution During Development of Bellis Perennis Ovules and Anthers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -

Establishing a Framework for Female Germline Initiation in the Plant Ovule Jorge Lora1, , Xiujuan Yang2, and Mathew R
Journal of Experimental Botany, Vol. 70, No. 11 pp. 2937–2949, 2019 doi:10.1093/jxb/erz212 Advance Access Publication 7 May 2019 Establishing a framework for female germline initiation in the plant ovule Jorge Lora1, , Xiujuan Yang2, and Mathew R. Tucker2,* 1 Department of Subtropical Fruits, Instituto de Hortofruticultura Subtropical y Mediterránea ‘La Mayora’ (IHSM-UMA-CSIC), 29750 Algarrobo-Costa, Málaga, Spain 2 School of Agriculture, Food and Wine, Waite Research Institute, The University of Adelaide, Glen Osmond, SA, Australia *Correspondence: [email protected] Received 27 November 2018; Editorial decision 29 April 2019; Accepted 2 May 2019 Editor: Sílvia Coimbra, University of Porto, Portugal Abstract Female gametogenesis in flowering plants initiates in the ovule, where a single germline progenitor differentiates from a pool of somatic cells. Germline initiation is a fundamental prerequisite for seed development but is poorly understood at the molecular level due to the location of the cells deep within the flower. Studies in Arabidopsis have shown that regulators of germline development include transcription factors such as NOZZLE/SPOROCYTELESS and WUSCHEL, components of the RNA-dependent DNA methylation pathway such as ARGONAUTE9 and RNADEPENDENT RNA POLYMERASE 6, and phytohormones such as auxin and cytokinin. These factors accumulate in a range of cell types from where they establish an environment to support germline differentiation. Recent studies provide fresh insight into the transition from somatic to germline identity, linking chromatin regulators, cell cycle genes, and novel mobile signals, capitalizing on cell type-specific methodologies in both dicot and monocot models. These findings are providing unique molecular and compositional insight into the mechanistic basis and evolutionary conservation of female germline development in plants. -

Structure and Function of Spores in the Aquatic Heterosporous Fern Family Marsileaceae
Int. J. Plant Sci. 163(4):485–505. 2002. ᭧ 2002 by The University of Chicago. All rights reserved. 1058-5893/2002/16304-0001$15.00 STRUCTURE AND FUNCTION OF SPORES IN THE AQUATIC HETEROSPOROUS FERN FAMILY MARSILEACEAE Harald Schneider1 and Kathleen M. Pryer2 Department of Botany, Field Museum of Natural History, 1400 South Lake Shore Drive, Chicago, Illinois 60605-2496, U.S.A. Spores of the aquatic heterosporous fern family Marsileaceae differ markedly from spores of Salviniaceae, the only other family of heterosporous ferns and sister group to Marsileaceae, and from spores of all ho- mosporous ferns. The marsileaceous outer spore wall (perine) is modified above the aperture into a structure, the acrolamella, and the perine and acrolamella are further modified into a remarkable gelatinous layer that envelops the spore. Observations with light and scanning electron microscopy indicate that the three living marsileaceous fern genera (Marsilea, Pilularia, and Regnellidium) each have distinctive spores, particularly with regard to the perine and acrolamella. Several spore characters support a division of Marsilea into two groups. Spore character evolution is discussed in the context of developmental and possible functional aspects. The gelatinous perine layer acts as a flexible, floating organ that envelops the spores only for a short time and appears to be an adaptation of marsileaceous ferns to amphibious habitats. The gelatinous nature of the perine layer is likely the result of acidic polysaccharide components in the spore wall that have hydrogel (swelling and shrinking) properties. Megaspores floating at the water/air interface form a concave meniscus, at the center of which is the gelatinous acrolamella that encloses a “sperm lake.” This meniscus creates a vortex-like effect that serves as a trap for free-swimming sperm cells, propelling them into the sperm lake. -

Megagametophyte Development of Crysanthemum Leuoanthemum L. Var. Pinnatifidum Lecoq. and Lamotte
Át1 A15MCT Q? ThE TIE$IS OF tohard ?artin for the M. 8. degree in otany Date thesis is prGented: May 14, 152 Title: ,gagarftetophyte 1evelopent of Cbrysantheuum Leuoantherniva L. var. pinnattfidw Lecoq. and L*aotte ibstraat approved: RedaCtedfOr PriVaCy Angiospern me.agarnetophytea are of three sain types; ¡onoeporic, bisporic, and tetrasortc. The nusber of weaspore nuclei taking part in seaametophyte deve1op nent te the detornining factor. In the first, only one of the tour me pore nuclei takes part in the development. in the second, two aegaspore nuclei take part in weagametophyte toration, and in the third, all tour nuclei contribute to the formation of the sature gasetophyte. In the sonosporto eight-nucleate, or Noraal type's, the seaapore aether cell nucleus undergoes the two reduottor divisions which result in the fornatton of a died and then a tetrad. The three atoropylar segasporee degenerate, and th nucleus of the one functional megaspore divides sitottoally to form a two-nucleate condition. Two further ttotic divisions fora a four-nucleate and an eight-nucleate stage. The asgagametophyte then differentiates a t)ree- celled egg apparatus, a primary endo.per oeil with two polar nuclei, and three antipodal oeils. In the tetraeporic sixteen-nucleate, or tDruea type", the nucleus of the megaepore mother cell undergoes the two reduotion divisions to form a single elongate cell oontaintn four segaspore nuolet. These then divide once attottoally to give rise to an eight-nucleate stage. )ne additional division produces a sixteen-nucleate weçagasetophyte which differentiates a three-coiled egg apparatus, a pritsary endoapers cell with to polar nuclei, arid eleven taninucleate antipodal oeils. -
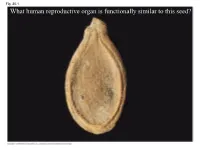
What Human Reproductive Organ Is Functionally Similar to This Seed?
Fig. 30-1 What human reproductive organ is functionally similar to this seed? Overview: Transforming the World • Seeds changed the course of plant evolution, enabling their bearers to become the dominant producers in most terrestrial ecosystems • A seed consists of an embryo and nutrients surrounded by a protective coat Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-1 What human reproductive organ is functionally similar to this seed? Seeds and pollen grains are key adaptations for life on land • In addition to seeds, the following are common to all seed plants – Reduced gametophytes – Heterospory – Ovules – Pollen Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Advantages of Reduced Gametophytes • The gametophytes of seed plants develop within the walls of spores that are retained within tissues of the parent sporophyte • Protect female gametophyte from UV and desiccation (good for the land life!) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-2 PLANT GROUP Mosses and other Ferns and other seedless Seed plants (gymnosperms and angiosperms) nonvascular plants vascular plants Reduced, independent Reduced (usually microscopic), dependent on surrounding (photosynthetic and Gametophyte Dominant sporophyte tissue for nutrition free-living) Sporophyte Reduced, dependent on Dominant Dominant gametophyte for nutrition Gymnosperm Angiosperm Sporophyte Microscopic female (2n) gametophytes (n) inside ovulate cone Microscopic Sporophyte -

Functional Investigation of Arabidopsis Callose Synthases and the Signal Transduction Pathway
FUNCTIONAL INVESTIGATION OF ARABIDOPSIS CALLOSE SYNTHASES AND THE SIGNAL TRANSDUCTION PATHWAY DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Xiaoyun Dong, M.S. (Plant Biology) The Ohio State University 2005 Dissertation Committee: Dr. Desh Pal S. Verma, Adviser Approved by Dr. Biao Ding _______________________________ Dr. Terry L. Graham Adviser Dr. Guo-liang Wang Graduate Program in Plant Pathology ABSTRACT Callose synthesis occurs at specific stages of cell wall development in all cell types, and in response to pathogen attack, wounding and physiological stresses. We isolated promoters of 12 Arabidopsis callose synthase (CalS1-12) genes and demonstrated that different callose synthases are expressed specifically in different tissues during plant development. That multiple CalS genes are expressed in the same cell type suggests the possibility that CalS complex may be constituted by heteromeric subunits. Five CalS genes were induced by pathogen (Peronospora parasitica, a causal agent of downy mildew) or salicylic acid (SA) treatments, while seven CalS genes were not affected by these treatments. Among the genes that are induced, CalS1 and CalS12, showed the highest responses. When expressed in npr1, a mutant impaired in the response of pathogen related (PR) genes to SA, the induction of CalS1 and CalS12 genes by the SA or pathogen treatments was significantly reduced. The patterns of expression of the other three CalS genes were not changed significantly in the npr1 mutant. These results suggest that the high induction observed of CalS1 and CalS12 is NPR1-dependent while the weak induction of all five CalS genes is NPR1-independent. -

Stratigraphic Distribution of Species of the Megaspore Genus Minerisporites in North America
Stratigraphic Distribution of Species of the Megaspore Genus Minerisporites in North America GEOLOGICAL SURVEY PROFESSIONAL PAPER 743-E Stratigraphic Distribution of Species of the Megaspore Genus Minerisporites in North America By ROBERT H. TSCHUDY CONTRIBUTIONS TO PALEONTOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 743-E Taxonomy, stratigraphic ranges, and facies significance of Minerisporites megaspores UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON: 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Tschudy, Robert H. Stratigraphic distribution of species of the megaspore genus Minerisporites in North America. (Contributions to Paleontology) (Geological Survey Professional Paper 743-E) Bibliography: p. Includes index. Supt. of Docs. no.: I 19.16:743-E 1. Minerisporites. 2. Paleobotany-Cretaceous. 3. Paleobotany-Paleocene. 4. Paleobotany-North America. I. Title. II. Series. III. Series: United States Geological Survey Professional Paper 743-E. QE996.T7 560' .13 76-608080 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock Number 024-001-02892-1 CONTENTS Page Page Abstract ............................................................................................... EI Systematics-Continued New species of Minerisporites............................................................ I Genus Minerisporites-Continued Stratigraphic distribution................................................................... I Minerisporites -

Ovule Ontogenesis and Megasporogenesis in Adesmia Latifolia (Spreng.) Vog
Revista Brasil. Bot., V.26, n.4, p.495-502, out.-dez. 2003 Ovule ontogenesis and megasporogenesis in Adesmia latifolia (Spreng.) Vog. (Leguminosae-Papilionoideae)1 MARIA C.C. MOÇO2,3 and JORGE E.A. MARIATH2 (received: January 29, 2003; accepted: September 11, 2003) ABSTRACT – (Ovule ontogenesis and megasporogenesis in Adesmia latifolia (Spreng.) Vog. (Leguminosae-Papilionoideae)). The ovule ontogenesis and the megasporogenesis events were studied under bright field, fluorescence and scanning electron microscopy. The primordium is 3-zonate and gives rise to a hemianatropous, bitegmic and crassinucellate ovule. The archesporium may consist of one or more archesporial cells, but only one undergoes meiosis, forming a linear tetrad. Normally, only a single megaspore is functional in the chalazal position, but occasionally two functional chalazal megaspores arise. The present study provides additional information on embryological characters in the Adesmieae tribe and discusses their taxonomic significance to the Leguminosae family. Key words - Adesmia, embryology, Leguminosae, megaspore, ovule RESUMO – (Ontogênese do óvulo e megasporogênese em Adesmia latifolia (Spreng.) Vog. (Leguminosae - Papilionoideae)). A ontogênese do óvulo e os eventos da megasporogênese foram estudados sob microscopia de campo claro, fluorescência e eletrônica de varredura. O primórdio é trizonado e origina um óvulo hemianátropo, bitegumentado e crassinucelado. O arquespório pode ser composto de mais de uma célula arquesporial, mas apenas uma sofre meiose formando uma tétrade linear. Normalmente, apenas um megásporo é funcional na posição calazal, mas foram registrados alguns casos em que os dois megásporos calazais eram, simultâneamente, funcionais. O presente estudo acrescenta informações sobre caracteres embriológicos na tribo Adesmieae e discute sua importância taxonômica na família Leguminosae. -

Morphological Studies Op Diploid Aud Autotetraploid
MORPHOLOGICAL STUDIES OP DIPLOID AUD AUTOTETRAPLOID PLARTS OP PHYSALIS PRUIUOSA L. Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By ROBERT DAVID HEURY, B.S., M.S. The Ohio State University 1958 Approved hy Department of Botany and Plant Pathology ACKN OWLEDGMEUT S The writer desires to take this opportunity to express his sincere thanks and appreciation to his adviser Dr, G, W. Blaydes for his guidance, advice, criticisms, and encouragement during the course of this investigation and preparation of the dissertation. Thanks are also extended to Dr. K. K. Pandey for his helpful suggestions concerning the research and to Mr. A. S. Heilman for the photographic work. ii TABLE OP COM'Ei'TTS Page IHTRODU CTI ON ...................................... 1 MATERIALS ARB M E T H O D S ........................ 4 GENERAL MORPHOLOGY AND G R O W T H ............... 8 Observations and Results ..................... 8 Discussion and Summary............ 21 PRE- ADD POST-PERTILIZATIOH MORPHOLOGY............. 27 Development of the Ovule and Emhryo Sac .... 27 Observations and Results ............. 27 Discussion and Summary ................... 33 Development of the Pollen ............ 37 Observations and Results ................. 37 Discussion and Summary ............... 42 Fertilization ........ ................. 43 Observations and Results • ••••...• 43 Discussion and Summary ............. 44 Endosperm ........ .............. 43 Observations and Results -

Gametophyte Development in Near the Interior Base
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Magazine R718 Primer tissues. These form multicellular The first gametophytic division bud-like structures, each of which is asymmetric, producing one develops into a leafy shoot. The large and one small cell. In C. mature gametophytes produce richardii, the large cell divides Gametophyte male and female sexual organs, again asymmetrically to produce development the antheridia and archegonia, another small cell, which later respectively. The gametophyte is develops into the rhizoid, a root- often sexually distinct, and plants like structure. The small cell from Wuxing Li and Hong Ma are either male or female. the first division becomes the Each antheridium has an outer protonemal initial, which divides Unlike animals, which produce layer that encloses and protects further to form a linear three- single-celled gametes directly thousands of motile sperm, which celled protonema. The middle cell from meiotic products, plants swim through available external then undergoes a transition in have generations which alternate water layer to the egg. Fertilization divisional plane, forming a two- between the diploid sporophyte at the base of the cylindrical dimensional structure. During and haploid gametophyte (Figure archegonium produces a diploid hermaphrodite development, a 1A). The diploid sporophytic zygote which develops into an meristem is formed in the two- generation develops from the unbranched sporophyte. The dimensional plane and gives rise zygote, the fusion product of sporophyte consists of a thin stalk to the male antheridia and the haploid gametes. Sporophytic attached to the gametophyte, and female archegonia. -

Lecture 7 Integrative Biology 168: Spring 2009
Key concepts -- Lecture 7 Integrative Biology 168: Spring 2009 INTRODUCTION TO SEED PLANTS Any understanding of the origin and morphology of the seed requires us to revisit the concept of heterospory (also see p. 103 in Simpson's Plant Systematics for diagram). Heterospory = production of two different types of spores (in two different types of sporangia): (1) megaspores (developing into female gametophytes = megagametophytes) and (2) microspores (developing into male gametophytes = microgametophytes). Sperm and egg are produced by different types of gametophytes in heterosporous plants. Each of the two principal free-sporing lineages of vascular plants (lycophytes and ferns, that is) have both homosporous and heterosporous taxa, with heterospory being independently derived from homospory in both groups. In heterosporous lycophytes and heterosporous ferns (as in homosporous lycophytes and ferns), the spore is the unit of dispersal and is released from the sporangium of the parent sporophyte prior to spore germination. From now on, we will be studying heterosporous plants that produce only one functional megaspore and retain it within the megasporangium -- the seed plants. All of the seed plants are heterosporous; the transition to heterospory from homospory happened before all characteristics of the seed evolved. [See p. 102, 104, and 105 of Simpson's Plant Systematics for diagrams that illustrate the following points] General points about evolution of seed: 1) Maternal protection and care by the sporophyte for the megagametophyte was taken to a new level in the seed plants; not only is the only functional megaspore retained in the megasporangium (of the sporophyte) but also the megagametophyte that germinates from that megaspore develops inside the megasporangium. -
Recent Advances in Understanding Female Gametophyte Development [Version 1; Peer Review: 2 Approved] Debra J Skinner1, Venkatesan Sundaresan 1,2
F1000Research 2018, 7(F1000 Faculty Rev):804 Last updated: 17 JUL 2019 REVIEW Recent advances in understanding female gametophyte development [version 1; peer review: 2 approved] Debra J Skinner1, Venkatesan Sundaresan 1,2 1Department of Plant Biology, University of California-Davis, Davis, USA 2Department of Plant Sciences, University of California-Davis, Davis, USA First published: 20 Jun 2018, 7(F1000 Faculty Rev):804 ( Open Peer Review v1 https://doi.org/10.12688/f1000research.14508.1) Latest published: 20 Jun 2018, 7(F1000 Faculty Rev):804 ( https://doi.org/10.12688/f1000research.14508.1) Reviewer Status Abstract Invited Reviewers The haploid female gametophyte (embryo sac) is an essential reproductive 1 2 unit of flowering plants, usually comprising four specialized cell types, including the female gametes (egg cell and central cell). The differentiation version 1 of these cells relies on spatial signals which pattern the gametophyte along published a proximal-distal axis, but the molecular and genetic mechanisms by which 20 Jun 2018 cell identities are determined in the embryo sac have long been a mystery. Recent identification of key genes for cell fate specification and their relationship to hormonal signaling pathways that act on positional cues has F1000 Faculty Reviews are written by members of provided new insights into these processes. A model for differentiation can the prestigious F1000 Faculty. They are be devised with egg cell fate as a default state of the female gametophyte commissioned and are peer reviewed before and with other cell types specified by the action of spatially regulated publication to ensure that the final, published version factors.