Gametophyte Development in Near the Interior Base
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -

Morphological Features of the Anther Development in Tomato Plants with Non-Specific Male Sterility
biology Article Morphological Features of the Anther Development in Tomato Plants with Non-Specific Male Sterility Inna A. Chaban 1, Neonila V. Kononenko 1 , Alexander A. Gulevich 1, Liliya R. Bogoutdinova 1, Marat R. Khaliluev 1,2,* and Ekaterina N. Baranova 1,* 1 All-Russia Research Institute of Agricultural Biotechnology, Timiryazevskaya 42, 127550 Moscow, Russia; [email protected] (I.A.C.); [email protected] (N.V.K.); [email protected] (A.A.G.); [email protected] (L.R.B.) 2 Moscow Timiryazev Agricultural Academy, Agronomy and Biotechnology Faculty, Russian State Agrarian University, Timiryazevskaya 49, 127550 Moscow, Russia * Correspondence: [email protected] (M.R.K.); [email protected] (E.N.B.) Received: 3 January 2020; Accepted: 12 February 2020; Published: 17 February 2020 Abstract: The study was devoted to morphological and cytoembryological analysis of disorders in the anther and pollen development of transgenic tomato plants with a normal and abnormal phenotype, which is characterized by the impaired development of generative organs. Various abnormalities in the structural organization of anthers and microspores were revealed. Such abnormalities in microspores lead to the blocking of asymmetric cell division and, accordingly, the male gametophyte formation. Some of the non-degenerated microspores accumulate a large number of storage inclusions, forming sterile mononuclear pseudo-pollen, which is similar in size and appearance to fertile pollen grain (looks like pollen grain). It was discussed that the growth of tapetal cells in abnormal anthers by increasing the size and ploidy level of nuclei contributes to this process. It has been shown that in transgenic plants with a normal phenotype, individual disturbances are also observed in the development of both male and female gametophytes. -

The Origin of Alternation of Generations in Land Plants
Theoriginof alternation of generations inlandplants: afocuson matrotrophy andhexose transport Linda K.E.Graham and LeeW .Wilcox Department of Botany,University of Wisconsin, 430Lincoln Drive, Madison,WI 53706, USA (lkgraham@facsta¡.wisc .edu ) Alifehistory involving alternation of two developmentally associated, multicellular generations (sporophyteand gametophyte) is anautapomorphy of embryophytes (bryophytes + vascularplants) . Microfossil dataindicate that Mid ^Late Ordovicianland plants possessed such alifecycle, and that the originof alternationof generationspreceded this date.Molecular phylogenetic data unambiguously relate charophyceangreen algae to the ancestryof monophyletic embryophytes, and identify bryophytes as early-divergentland plants. Comparison of reproduction in charophyceans and bryophytes suggests that the followingstages occurredduring evolutionary origin of embryophytic alternation of generations: (i) originof oogamy;(ii) retention ofeggsand zygotes on the parentalthallus; (iii) originof matrotrophy (regulatedtransfer ofnutritional and morphogenetic solutes fromparental cells tothe nextgeneration); (iv)origin of a multicellularsporophyte generation ;and(v) origin of non-£ agellate, walled spores. Oogamy,egg/zygoteretention andmatrotrophy characterize at least some moderncharophyceans, and arepostulated to represent pre-adaptativefeatures inherited byembryophytes from ancestral charophyceans.Matrotrophy is hypothesizedto have preceded originof the multicellularsporophytes of plants,and to represent acritical innovation.Molecular -

Seedless Plants Key Concept Seedless Plants Do Not Produce Seeds 2 but Are Well Adapted for Reproduction and Survival
Seedless Plants Key Concept Seedless plants do not produce seeds 2 but are well adapted for reproduction and survival. What You Will Learn When you think of plants, you probably think of plants, • Nonvascular plants do not have such as trees and flowers, that make seeds. But two groups of specialized vascular tissues. plants don’t make seeds. The two groups of seedless plants are • Seedless vascular plants have specialized vascular tissues. nonvascular plants and seedless vascular plants. • Seedless plants reproduce sexually and asexually, but they need water Nonvascular Plants to reproduce. Mosses, liverworts, and hornworts do not have vascular • Seedless plants have two stages tissue to transport water and nutrients. Each cell of the plant in their life cycle. must get water from the environment or from a nearby cell. So, Why It Matters nonvascular plants usually live in places that are damp. Also, Seedless plants play many roles in nonvascular plants are small. They grow on soil, the bark of the environment, including helping to form soil and preventing erosion. trees, and rocks. Mosses, liverworts, and hornworts don’t have true stems, roots, or leaves. They do, however, have structures Vocabulary that carry out the activities of stems, roots, and leaves. • rhizoid • rhizome Mosses Large groups of mosses cover soil or rocks with a mat of Graphic Organizer In your Science tiny green plants. Mosses have leafy stalks and rhizoids. A Journal, create a Venn Diagram that rhizoid is a rootlike structure that holds nonvascular plants in compares vascular plants and nonvas- place. Rhizoids help the plants get water and nutrients. -

Microsporogenesis and Male Gametogenesis in Jatropha Curcas L. (Euphorbiaceae)1 Huanfang F
Journal of the Torrey Botanical Society 134(3), 2007, pp. 335–343 Microsporogenesis and male gametogenesis in Jatropha curcas L. (Euphorbiaceae)1 Huanfang F. Liu South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, China, and Graduate School of Chinese Academy of Sciences, Beijing, 100039, China Bruce K. Kirchoff University of North Carolina at Greensboro, Department of Biology, 312 Eberhart, P.O. Box 26170, Greensboro, NC 27402-6170 Guojiang J. Wu and Jingping P. Liao2 South China Botanical Garden, Chinese Academy of Sciences, Key Laboratory of Digital Botanical Garden in Guangdong, Guangzhou, 510650, China LIU, H. F. (South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, China, and Graduate School of Chinese Academy of Sciences, Beijing, 100039, China), B. K. KIRCHOFF (University of North Carolina at Greensboro, Department of Biology, 312 Eberhart, P.O. Box 26170, Greensboro, NC 27402-6170), G. J. WU, AND J. P. LIAO (South China Botanical Garden, Chinese Academy of Sciences, Key Laboratory of Digital Botanical Garden in Guangdong, Guangzhou, 510650, China). Microsporogenesis and male gametogenesis in Jatropha curcas L. (Euphorbiaceae). J. Torrey Bot. Soc. 134: 335–343. 2007.— Microsporogenesis and male gametogenesis of Jatropha curcas L. (Euphorbiaceae) was studied in order to provide additional data on this poorly studied family. Male flowers of J. curcas have ten stamens, which each bear four microsporangia. The development of the anther wall is of the dicotyledonous type, and is composed of an epidermis, endothecium, middle layer(s) and glandular tapetum. The cytokinesis following meiosis is simultaneous, producing tetrahedral tetrads. Mature pollen grains are two-celled at anthesis, with a spindle shaped generative cell. -

Mosses and Ferns
Mosses and Ferns • How did they evolve from Protists? Moss and Fern Life Cycles Group 1: Seedless, Nonvascular Plants • Live in moist environments to reproduce • Grow low to ground to retain moisture (nonvascular) • Lack true leaves • Common pioneer species during succession • Gametophyte most common (dominant) • Ex: Mosses, liverworts, hornworts Moss Life Cycle 1)Moss 2) Through water, 3) Diploid sporophyte 4) Sporophyte will gametophytes sperm from the male will grow from zygote create and release grow near the gametophyte will haploid spores ground swim to the female (haploid stage) gametophyte to create a diploid zygote Diploid sporophyte . zygo egg zygo te egg te zygo zygo egg egg te te male male female female female male female male Haploid gametophytes 5) Haploid 6) The process spores land repeats and grow into new . gametophytes . Haploid gametophytesground . sporophyte . zygo egg zygo te egg te zygo zygo egg egg te te male male female female female male female male Haploid gametophytes • Vascular system allows Group 2: Seedless, – Taller growth – Nutrient transportation Vascular Plants • Live in moist environments – swimming sperm • Gametophyte stage – Male gametophyte: makes sperm – Female gametophyte: makes eggs – Sperm swims to fertilize eggs • Sporophyte stage – Spores released into air – Spores land and grow into gametophyte • Ex: Ferns, Club mosses, Horsetails Fern Life Cycle 1) Sporophyte creates and releases haploid spores Adult Sporophyte . ground 2) Haploid spores land in the soil . ground 3) From the haploid spores, gametophyte grows in the soil Let’s zoom in Fern gametophytes are called a prothallus ground 4) Sperm swim through water from the male parts (antheridium) to the female parts (archegonia)…zygote created Let’s zoom back out zygo zygo egg egg te te zygo egg te 5) Diploid sporophyte grows from the zygote sporophyte Fern gametophytes are called a prothallus ground 6) Fiddle head uncurls….fronds open up 7) Cycle repeats -- Haploid spores created and released . -

Plant Reproduction
AccessScience from McGraw-Hill Education Page 1 of 10 www.accessscience.com Plant reproduction Contributed by: Scott D. Russell Publication year: 2014 The formation of a new plant that is either an exact copy or recombination of the genetic makeup of its parents. There are three types of plant reproduction considered here: (1) vegetative reproduction, in which a vegetative organ forms a clone of the parent; (2) asexual reproduction, in which reproductive components undergo a nonsexual form of production of offspring without genetic rearrangement, also known as apomixis; and (3) sexual reproduction, in which meiosis (reduction division) leads to formation of male and female gametes that combine through syngamy (union of gametes) to produce offspring. See also: PLANT; PLANT PHYSIOLOGY. Vegetative reproduction Unlike animals, plants may be readily stimulated to produce identical copies of themselves through cloning. In animals, only a few cells, which are regarded as stem cells, are capable of generating cell lineages, organs, or new organisms. In contrast, plants generate or produce stem cells from many plant cells of the root, stem, or leaf that are not part of an obvious generative lineage—a characteristic that has been known as totipotency, or the general ability of a single cell to regenerate a whole new plant. This ability to establish new plants from one or more cells is the foundation of plant biotechnology. In biotechnology, a single cell may be used to regenerate new organisms that may or may not genetically differ from the original organism. If it is identical to the parent, it is a clone; however, if this plant has been altered through molecular biology, it is known as a genetically modified organism (GMO). -

External Morphological Characteristics for Periods During Pecan Microspore and Pollen Differentiation I.E
J. AMER. Soc. HORT. SCI. 117(1):190-196. 1992. External Morphological Characteristics for Periods during Pecan Microspore and Pollen Differentiation I.E. Yates Russell Research Center, Agricultural Research Service, U.S. Department of Agriculture, Athens, GA 30613 Darrell Sparks Department of Horticulture, University of Georgia, Athens, GA 30602 Additional index words. Carya illinoensis, staminate flower, meiosis, cytology, cytochemistry, electron microscopy Abstract. Catkin external morphological characteristics of a protogynous (’Stuart’) and a protandrous (’Desirable’) cultivar of pecan [Carya illinoensis (Wangenh.) C. Koch] were related temporally to the differentiation of microspore and pollen grains. Reproductive cell development was divided into seven periods based on evaluations of number, location, and intensity of staining of the nucleus and/or nucleolus; and vacuolization and staining intensity of the cytoplasm. Catkins with anthers and bracteoles enclosed by bracts did not have reproductive cells that were matured to free microspore. Free microspore developed only after bracteoles became externally visible. The Period 1 nucleus was at the periphery of the cell and a large central vacuole was present; at Period 2, the nucleus was at the center and vacuolation had been reduced. As the angle between the bract and catkin rachis increased to 45°, vacnolation was reduced as the nucleus enlarged and moved to a central location in the microspore (Periods 3 and 4). The majority of the pollen grains were binucleate, and the generative nucleus became elliptical (Periods 5 and 6) by the time anthers became externally visible. Acetocarmine staining intensity of cellular components masked the presence of the gener- ative nucleus (Period 7) just before anther dehiscence. -

Structure and Function of Spores in the Aquatic Heterosporous Fern Family Marsileaceae
Int. J. Plant Sci. 163(4):485–505. 2002. ᭧ 2002 by The University of Chicago. All rights reserved. 1058-5893/2002/16304-0001$15.00 STRUCTURE AND FUNCTION OF SPORES IN THE AQUATIC HETEROSPOROUS FERN FAMILY MARSILEACEAE Harald Schneider1 and Kathleen M. Pryer2 Department of Botany, Field Museum of Natural History, 1400 South Lake Shore Drive, Chicago, Illinois 60605-2496, U.S.A. Spores of the aquatic heterosporous fern family Marsileaceae differ markedly from spores of Salviniaceae, the only other family of heterosporous ferns and sister group to Marsileaceae, and from spores of all ho- mosporous ferns. The marsileaceous outer spore wall (perine) is modified above the aperture into a structure, the acrolamella, and the perine and acrolamella are further modified into a remarkable gelatinous layer that envelops the spore. Observations with light and scanning electron microscopy indicate that the three living marsileaceous fern genera (Marsilea, Pilularia, and Regnellidium) each have distinctive spores, particularly with regard to the perine and acrolamella. Several spore characters support a division of Marsilea into two groups. Spore character evolution is discussed in the context of developmental and possible functional aspects. The gelatinous perine layer acts as a flexible, floating organ that envelops the spores only for a short time and appears to be an adaptation of marsileaceous ferns to amphibious habitats. The gelatinous nature of the perine layer is likely the result of acidic polysaccharide components in the spore wall that have hydrogel (swelling and shrinking) properties. Megaspores floating at the water/air interface form a concave meniscus, at the center of which is the gelatinous acrolamella that encloses a “sperm lake.” This meniscus creates a vortex-like effect that serves as a trap for free-swimming sperm cells, propelling them into the sperm lake. -

Megagametophyte Development of Crysanthemum Leuoanthemum L. Var. Pinnatifidum Lecoq. and Lamotte
Át1 A15MCT Q? ThE TIE$IS OF tohard ?artin for the M. 8. degree in otany Date thesis is prGented: May 14, 152 Title: ,gagarftetophyte 1evelopent of Cbrysantheuum Leuoantherniva L. var. pinnattfidw Lecoq. and L*aotte ibstraat approved: RedaCtedfOr PriVaCy Angiospern me.agarnetophytea are of three sain types; ¡onoeporic, bisporic, and tetrasortc. The nusber of weaspore nuclei taking part in seaametophyte deve1op nent te the detornining factor. In the first, only one of the tour me pore nuclei takes part in the development. in the second, two aegaspore nuclei take part in weagametophyte toration, and in the third, all tour nuclei contribute to the formation of the sature gasetophyte. In the sonosporto eight-nucleate, or Noraal type's, the seaapore aether cell nucleus undergoes the two reduottor divisions which result in the fornatton of a died and then a tetrad. The three atoropylar segasporee degenerate, and th nucleus of the one functional megaspore divides sitottoally to form a two-nucleate condition. Two further ttotic divisions fora a four-nucleate and an eight-nucleate stage. The asgagametophyte then differentiates a t)ree- celled egg apparatus, a primary endo.per oeil with two polar nuclei, and three antipodal oeils. In the tetraeporic sixteen-nucleate, or tDruea type", the nucleus of the megaepore mother cell undergoes the two reduotion divisions to form a single elongate cell oontaintn four segaspore nuolet. These then divide once attottoally to give rise to an eight-nucleate stage. )ne additional division produces a sixteen-nucleate weçagasetophyte which differentiates a three-coiled egg apparatus, a pritsary endoapers cell with to polar nuclei, arid eleven taninucleate antipodal oeils. -
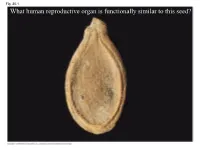
What Human Reproductive Organ Is Functionally Similar to This Seed?
Fig. 30-1 What human reproductive organ is functionally similar to this seed? Overview: Transforming the World • Seeds changed the course of plant evolution, enabling their bearers to become the dominant producers in most terrestrial ecosystems • A seed consists of an embryo and nutrients surrounded by a protective coat Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-1 What human reproductive organ is functionally similar to this seed? Seeds and pollen grains are key adaptations for life on land • In addition to seeds, the following are common to all seed plants – Reduced gametophytes – Heterospory – Ovules – Pollen Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Advantages of Reduced Gametophytes • The gametophytes of seed plants develop within the walls of spores that are retained within tissues of the parent sporophyte • Protect female gametophyte from UV and desiccation (good for the land life!) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-2 PLANT GROUP Mosses and other Ferns and other seedless Seed plants (gymnosperms and angiosperms) nonvascular plants vascular plants Reduced, independent Reduced (usually microscopic), dependent on surrounding (photosynthetic and Gametophyte Dominant sporophyte tissue for nutrition free-living) Sporophyte Reduced, dependent on Dominant Dominant gametophyte for nutrition Gymnosperm Angiosperm Sporophyte Microscopic female (2n) gametophytes (n) inside ovulate cone Microscopic Sporophyte