Lab 14: Qualitative Organic Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
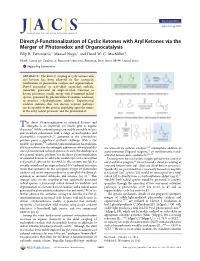
Direct Β‑Functionalization of Cyclic Ketones with Aryl Ketones Via the Merger of Photoredox and Organocatalysis Filip R
Communication pubs.acs.org/JACS Direct β‑Functionalization of Cyclic Ketones with Aryl Ketones via the Merger of Photoredox and Organocatalysis Filip R. Petronijevic,́† Manuel Nappi,† and David W. C. MacMillan* Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States *S Supporting Information ABSTRACT: The direct β-coupling of cyclic ketones with aryl ketones has been achieved via the synergistic combination of photoredox catalysis and organocatalysis. Diaryl oxymethyl or aryl−alkyl oxymethyl radicals, transiently generated via single-electron reduction of ketone precursors, readily merge with β-enaminyl radical species, generated by photon-induced enamine oxidation, to produce γ-hydroxyketone adducts. Experimental evidence indicates that two discrete reaction pathways can be operable in this process depending upon the nature of the ketyl radical precursor and the photocatalyst. he direct β-functionalization of saturated ketones and T aldehydes is an important yet elusive goal in organic chemistry.1 While carbonyl groups are readily amenable to ipso- and α-carbon substitution with a range of nucleophiles and electrophiles respectively,2,3 activation at the β-methylene position poses a significant synthetic challenge. With a few notable exceptions,1,4 carbonyl β-functionalization has tradition- ally been restricted to the conjugate addition of soft nucleophiles are accessed via carbene catalysis,9,10 nucleophilic addition of into α,β-unsaturated carbonyl systems. As such, the development acetal-protected Grignard reagents,11 or stoichiometric metal- 5 − of a general catalytic platform for the direct β-functionalization activated homoenolate equivalents.12 16 of saturated ketones or aldehydes would represent a conceptual Drawing from the mechanistic insights gained in the course of and practical advance for the field. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

Aldehydes and Ketones Are Simple Organic Compounds Containing a Carbonyl Group
Aldehydes and Ketones are simple organic compounds containing a carbonyl group. Carbonyl group contains carbon- oxygen double bond. These organic compounds are simple because the carbon atom presents in the carbonyl group lack reactive groups such as OH or Cl. By Dr. Sayed Hasan Mehdi Assistant Professor Department of Chemistry Shia P.G. College, Lucknow Dr. S.Hasan Mehdi 6/13/2020 This is to bring to kind notice that the matter of this e- content is for the B.Sc. IV semester students. It has been taken from the following sources. The students are advised to follow these books as well. •A TEXTBOOK OF ORGANIC CHEMISTRY by Arun Bahl & B.S. Bahl, S. Chand & Company Ltd. Publication. •Graduate Organic Chemistry by M. K. Jain and S.C. Sharma, Vishal Publishing Co. •Pradeep’s Organic Chemistry Vol II by R. N. Dhawan, Pradeep Publication, Jalandhar. Dr. S.Hasan Mehdi 6/13/2020 An aldehyde is one of the classes of carbonyl group- containing alkyl group on one end and hydrogen on the other end. The R and Ar denote alkyl or aryl member respectively. In the condensed form, the aldehyde is written as –CHO. Dr. S.Hasan Mehdi 6/13/2020 Dr. S.Hasan Mehdi 6/13/2020 1. From Alcohols: a. By oxidation of Alcohols: Aldehydes and ketones can be prepared by the controlled oxidation of primary and secondary alcohols using an acidified solution of potassium dichromate or permanganate. Primary alcohol produces aldehydesRef. Last slide. O K Cr O RCH2OH + [O] 2 2 7 + R C H H 10 Alcohol Aldehyde O CH3CH2OH+ [O] K2Cr2O7 + CH3 C H H Ethyl Alcohol Acetaldehyde The aldehydes formed in the above reaction are very easily oxidised to carboxylc acids if allowed to remain in the reaction mixture. -

EPA Method 8315A (SW-846): Determination of Carbonyl Compounds by High Performance Liquid Chromatography (HPLC)
METHOD 8315A DETERMINATION OF CARBONYL COMPOUNDS BY HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) 1.0 SCOPE AND APPLICATION 1.1 This method provides procedures for the determination of free carbonyl compounds in various matrices by derivatization with 2,4-dinitrophenylhydrazine (DNPH). The method utilizes high performance liquid chromatography (HPLC) with ultraviolet/visible (UV/vis) detection to identify and quantitate the target analytes. This method includes two procedures encompassing all aspects of the analysis (extraction to determination of concentration). Procedure 1 is appropriate for the analysis of aqueous, soil and waste samples and stack samples collected by Method 0011. Procedure 2 is appropriate for the analysis of indoor air samples collected by Method 0100. The list of target analytes differs by procedure. The appropriate procedure for each target analyte is listed in the table below. Compound CAS No. a Proc. 1b Proc. 2 b Acetaldehyde 75-07-0 X X Acetone 67-64-1 X Acrolein 107-02-8 X Benzaldehyde 100-52-7 X Butanal (Butyraldehyde) 123-72-8 X X Crotonaldehyde 123-73-9 X X Cyclohexanone 108-94-1 X Decanal 112-31-2 X 2,5-Dimethylbenzaldehyde 5779-94-2 X Formaldehyde 50-00-0 X X Heptanal 111-71-7 X Hexanal (Hexaldehyde) 66-25-1 X X Isovaleraldehyde 590-86-3 X Nonanal 124-19-6 X Octanal 124-13-0 X Pentanal (Valeraldehyde) 110-62-3 X X Propanal (Propionaldehyde) 123-38-6 X X m-Tolualdehyde 620-23-5 X X o-Tolualdehyde 529-20-4 X p-Tolualdehyde 104-87-0 X a Chemical Abstract Service Registry Number. -

Nitroso and Nitro Compounds 11/22/2014 Part 1
Hai Dao Baran Group Meeting Nitroso and Nitro Compounds 11/22/2014 Part 1. Introduction Nitro Compounds O D(Kcal/mol) d (Å) NO NO+ Ph NO Ph N cellular signaling 2 N O N O OH CH3−NO 40 1.48 molecule in mammals a nitro compound a nitronic acid nitric oxide b.p = 100 oC (8 mm) o CH3−NO2 57 1.47 nitrosonium m.p = 84 C ion (pKa = 2−6) CH3−NH2 79 1.47 IR: υ(N=O): 1621-1539 cm-1 CH3−I 56 Nitro group is an EWG (both −I and −M) Reaction Modes Nitro group is a "sink" of electron Nitroso vs. olefin: e Diels-Alder reaction: as dienophiles Nu O NO − NO Ene reaction 3 2 2 NO + N R h 2 O e Cope rearrangement υ O O Nu R2 N N N R1 N Nitroso vs. carbonyl R1 O O O O O N O O hυ Nucleophilic addition [O] N R2 R O O R3 Other reaction modes nitrite Radical addition high temp low temp nitrolium EWG [H] ion brown color less ion Redox reaction Photochemical reaction Nitroso Compounds (C-Nitroso Compounds) R2 R1 O R3 R1 Synthesis of C-Nitroso Compounds 2 O R1 R 2 N R3 3 R 3 N R N R N 3 + R2 2 R N O With NO sources: NaNO2/HCl, NOBF4, NOCl, NOSbF6, RONO... 1 R O R R1 O Substitution trans-dimer monomer: blue color cis-dimer colorless colorless R R NOBF OH 4 - R = OH, OMe, Me, NR2, NHR N R2 R3 = H or NaNO /HCl - para-selectivity ΔG = 10 Kcal mol-1 Me 2 Me R1 NO oxime R rate determining step Blue color: n π∗ absorption band 630-790 nm IR: υ(N=O): 1621-1539 cm-1, dimer υ(N−O): 1300 (cis), 1200 (trans) cm-1 + 1 Me H NMR (α-C-H) δ = 4 ppm: nitroso is an EWG ON H 3 Kochi et al. -

Comparative Study of Disinfectants for Use in Low-Cost Gravity Driven Household Water Purifiers Rajshree A
443 © IWA Publishing 2013 Journal of Water and Health | 11.3 | 2013 Comparative study of disinfectants for use in low-cost gravity driven household water purifiers Rajshree A. Patil, Shankar B. Kausley, Pradeep L. Balkunde and Chetan P. Malhotra ABSTRACT Point-of-use (POU) gravity-driven household water purifiers have been proven to be a simple, low- Rajshree A. Patil Shankar B. Kausley (corresponding author) cost and effective intervention for reducing the impact of waterborne diseases in developing Pradeep L. Balkunde Chetan P. Malhotra countries. The goal of this study was to compare commonly used water disinfectants for their TCS Innovation Labs – TRDDC, fi 54B, Hadapsar Industrial Estate, feasibility of adoption in low-cost POU water puri ers. The potency of each candidate disinfectant Pune - 411013, was evaluated by conducting a batch disinfection study for estimating the concentration of India E-mail: [email protected] disinfectant needed to inactivate a given concentration of the bacterial strain Escherichia coli ATCC 11229. Based on the concentration of disinfectant required, the size, weight and cost of a model purifier employing that disinfectant were estimated. Model purifiers based on different disinfectants were compared and disinfectants which resulted in the most safe, compact and inexpensive purifiers were identified. Purifiers based on bromine, tincture iodine, calcium hypochlorite and sodium dichloroisocyanurate were found to be most efficient, cost effective and compact with replacement parts costing US$3.60–6.00 for every 3,000 L of water purified and are thus expected to present the most attractive value proposition to end users. Key words | disinfectant, drinking water, E. -

(12) United States Patent (10) Patent No.: US 7.494,962 B2 Kinet Al
USOO74949.62B2 (12) United States Patent (10) Patent No.: US 7.494,962 B2 Kinet al. (45) Date of Patent: Feb. 24, 2009 (54) SOLVENTS CONTAINING CYCLOAKYL (56) References Cited ALKYLETHERS AND PROCESS FOR PRODUCTION OF THE ETHERS U.S. PATENT DOCUMENTS 3496,223 A * 2/1970 Mitchell et al. ............... 562/22 (75) Inventors: Idan Kin, Ottawa (CA); Genichi Ohta, Tokyo (JP); Kazuo Teraishi, Tokyo (JP); Kiyoshi Watanabe, Tokyo (JP) (Continued) (73) Assignee: Zeon Corporation, Tokyo (JP) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this EP 587434 A1 3, 1994 patent is extended or adjusted under 35 U.S.C. 154(b) by 349 days. (Continued) (21) Appl. No.: 10/481,340 OTHER PUBLICATIONS (22) PCT Filed: Jun. 27, 2002 Edited by Kagaku Daijiten Henshu Iinkai, “Kagaku Daijiten 9”. (86). PCT No.: PCT/UP02/06501 Kyoritsu Shuppan Co., Ltd., Aug. 25, 1962, p. 437, "Yozai'. (Continued) S371 (c)(1), (2), (4) Date: Sep. 24, 2004 Primary Examiner Gregory E Webb (74) Attorney, Agent, or Firm—Birch, Stewart, Kolasch & (87) PCT Pub. No.: WO03/002500 Birch, LLP PCT Pub. Date: Jan. 9, 2003 (57) ABSTRACT (65) Prior Publication Data The present inventions are (A) a solvent comprising at least US 2005/OO65060A1 Mar. 24, 2005 one cycloalkyl alkyl ether (1) represented by the general O O formula: R1-O R2 (wherein R1 is cyclopentyl or the like: (30) Foreign Application Priority Data and R2 is C1-10 alkyl or the like); (B) a method of prepara Jun. 28, 2001 (JP) ............................. 2001-196766 tions the ethers (1) characterized by reacting an alicyclic Oct. -

Silver Nanoparticle Nanocomposite Filaments Produced by an In-Situ Reduction Reactive Melt Mixing Process
biomimetics Article Three-Dimensional Printed Antimicrobial Objects of Polylactic Acid (PLA)-Silver Nanoparticle Nanocomposite Filaments Produced by an In-Situ Reduction Reactive Melt Mixing Process Nectarios Vidakis 1, Markos Petousis 1,* , Emmanouel Velidakis 1, Marco Liebscher 2 and Lazaros Tzounis 3 1 Mechanical Engineering Department, Hellenic Mediterranean University, Estavromenos, 71004 Heraklion, Crete, Greece; [email protected] (N.V.); [email protected] (E.V.) 2 Institute of Construction Materials, Technische Universität Dresden, DE-01062 Dresden, Germany; [email protected] 3 Department of Materials Science and Engineering, University of Ioannina, 45110 Ioannina, Greece; [email protected] * Correspondence: [email protected]; Tel.: +30-2810-37-9227 Received: 14 August 2020; Accepted: 31 August 2020; Published: 2 September 2020 Abstract: In this study, an industrially scalable method is reported for the fabrication of polylactic acid (PLA)/silver nanoparticle (AgNP) nanocomposite filaments by an in-situ reduction reactive melt mixing method. The PLA/AgNP nanocomposite filaments have been produced initially + reducing silver ions (Ag ) arising from silver nitrate (AgNO3) precursor mixed in the polymer melt to elemental silver (Ag0) nanoparticles, utilizing polyethylene glycol (PEG) or polyvinyl pyrrolidone (PVP), respectively, as macromolecular blend compound reducing agents. PEG and PVP were added at various concentrations, to the PLA matrix. The PLA/AgNP filaments have been used to manufacture 3D printed antimicrobial (AM) parts by Fused Filament Fabrication (FFF). The 3D printed PLA/AgNP parts exhibited significant AM properties examined by the reduction in Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) bacteria viability (%) experiments at 30, 60, and 120 min duration of contact (p < 0.05; p-value (p): probability). -

Syntheses and Eliminations of Cyclopentyl Derivatives David John Rausch Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1966 Syntheses and eliminations of cyclopentyl derivatives David John Rausch Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Rausch, David John, "Syntheses and eliminations of cyclopentyl derivatives " (1966). Retrospective Theses and Dissertations. 2875. https://lib.dr.iastate.edu/rtd/2875 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 66—6996 RAUSCH, David John, 1940- SYNTHESES AND ELIMINATIONS OF CYCLOPENTYL DERIVATIVES. Iowa State University of Science and Technology Ph.D., 1966 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan SYNTHESES AND ELIMINATIONS OF CYCLOPENTYL DERIVATIVES by David John Rausch A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved : Signature was redacted for privacy. Signature was redacted for privacy. Head of Major Department Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1966 ii TABLE OF CONTENTS VITA INTRODUCTION HISTORICAL Conformation of Cyclopentanes Elimination Reactions RESULTS AND DISCUSSION Synthetic Elimination Reactions EXPERIMENTAL Preparation and Purification of Materials Procedures and Data for Beta Elimination Reactions SUMMARY LITERATURE CITED ACKNOWLEDGEMENTS iii VITA The author was born in Aurora, Illinois, on October 24, 1940, to Mr. -

Chapter 14 – Aldehydes and Ketones
Chapter 14 – Aldehydes and Ketones 14.1 Structures and Physical Properties of Aldehydes and Ketones Ketones and aldehydes are related in that they each possess a C=O (carbonyl) group. They differ in that the carbonyl carbon in ketones is bound to two carbon atoms (RCOR’), while that in aldehydes is bound to at least one hydrogen (H2CO and RCHO). Thus aldehydes always place the carbonyl group on a terminal (end) carbon, while the carbonyl group in ketones is always internal. Some common examples include (common name in parentheses): O O H HH methanal (formaldehyde) trans-3-phenyl-2-propenal (cinnamaldehyde) preservative oil of cinnamon O O propanone (acetone) 3-methylcyclopentadecanone (muscone) nail polish remover a component of one type of musk oil Simple aldehydes (e.g. formaldehyde) typically have an unpleasant, irritating odor. Aldehydes adjacent to a string of double bonds (e.g. 3-phenyl-2-propenal) frequently have pleasant odors. Other examples include the primary flavoring agents in oil of bitter almond (Ph- CHO) and vanilla (C6H3(OH)(OCH3)(CHO)). As your book says, simple ketones have distinctive odors (similar to acetone) that are typically not unpleasant in low doses. Like aldehydes, placing a collection of double bonds adjacent to a ketone carbonyl generally makes the substance more fragrant. The primary flavoring agent in oil of caraway is just a such a ketone. 2 O oil of carraway Because the C=O group is polar, small aldehydes and ketones enjoy significant water solubility. They are also quite soluble in typical organic solvents. 14.2 Naming Aldehydes and Ketones Aldehydes The IUPAC names for aldehydes are obtained by using rules similar to those we’ve seen for other functional groups (e.g. -

Synthetic Turf Scientific Advisory Panel Meeting Materials
California Environmental Protection Agency Office of Environmental Health Hazard Assessment Synthetic Turf Study Synthetic Turf Scientific Advisory Panel Meeting May 31, 2019 MEETING MATERIALS THIS PAGE LEFT BLANK INTENTIONALLY Office of Environmental Health Hazard Assessment California Environmental Protection Agency Agenda Synthetic Turf Scientific Advisory Panel Meeting May 31, 2019, 9:30 a.m. – 4:00 p.m. 1001 I Street, CalEPA Headquarters Building, Sacramento Byron Sher Auditorium The agenda for this meeting is given below. The order of items on the agenda is provided for general reference only. The order in which items are taken up by the Panel is subject to change. 1. Welcome and Opening Remarks 2. Synthetic Turf and Playground Studies Overview 4. Synthetic Turf Field Exposure Model Exposure Equations Exposure Parameters 3. Non-Targeted Chemical Analysis Volatile Organics on Synthetic Turf Fields Non-Polar Organics Constituents in Crumb Rubber Polar Organic Constituents in Crumb Rubber 5. Public Comments: For members of the public attending in-person: Comments will be limited to three minutes per commenter. For members of the public attending via the internet: Comments may be sent via email to [email protected]. Email comments will be read aloud, up to three minutes each, by staff of OEHHA during the public comment period, as time allows. 6. Further Panel Discussion and Closing Remarks 7. Wrap Up and Adjournment Agenda Synthetic Turf Advisory Panel Meeting May 31, 2019 THIS PAGE LEFT BLANK INTENTIONALLY Office of Environmental Health Hazard Assessment California Environmental Protection Agency DRAFT for Discussion at May 2019 SAP Meeting. Table of Contents Synthetic Turf and Playground Studies Overview May 2019 Update .....