Application No. AU 2020210286 Al (19) AUSTRALIAN PATENT OFFICE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States July 2016 2 Table of Contents
Deuterium Labelled Compounds United States July 2016 2 Table of Contents International Distributors 3 Corporate Overview 4 General Information 5 Pricing and Payment 5 Quotations 5 Custom Synthesis 5 Shipping 5 Quality Control 6 Quotations 6 Custom Synthesis 6 Shipping 6 Quality Control 6 Chemical Abstract Service Numbers 6 Handling Hazardous Compounds 6 Our Products are Not Intended for Use in Humans 7 Limited Warranty 7 Packaging Information 7 Alphabetical Listings 8 Stock Clearance 236 Products by Category 242 n-Alkanes 243 α-Amino Acids, N-Acyl α-Amino Acids, N-t-BOC Protected α-Amino Acid 243 and N-FMOC Protected α-Amino Acids Buffers and Reagents for NMR Studies 245 Detergents 245 Environmental Standards 246 Fatty Acids and Fatty Acid Esters 249 Flavours and Fragrances 250 Gases 253 Medical Research Products 254 Nucleic Acid Bases and Nucleosides 255 Pesticides and Pesticide Metabolites 256 Pharmaceutical Standards 257 Polyaromatic Hydrocarbons (PAHs), Alkyl-PAHs, Amino-PAHs, 260 Hydroxy-PAHs and Nitro-PAHs Polychlorinated Biphenyls (PCBs) 260 Spin Labels 261 Steroids 261 3 International Distributors C Beijng Zhenxiang H EQ Laboratories GmbH Australia K Technology Company Graf-von-Seyssel-Str. 10 Rm. 15A01, Changyin Bld. 86199 Augsburg Austria H No. 88, YongDingLu Rd. Germany Beijing 100039 Tel.: (49) 821 71058246 Belgium J China Fax: (49) 821 71058247 Tel.: (86) 10-58896805 [email protected] China C Fax: (86) 10-58896158 www.eqlabs.de Czech Republic H [email protected] Germany, Austria, China Czech Republic, Greece, Denmark I Hungary, -

Campro Catalog Stable Isotope
Introduction & Welcome Dear Valued Customer, We are pleased to present to you our Stable Isotopes Catalog which contains more than three thousand (3000) high quality labeled compounds. You will find new additions that are beneficial for your research. Campro Scientific is proud to work together with Isotec, Inc. for the distribution and marketing of their stable isotopes. We have been working with Isotec for more than twenty years and know that their products meet the highest standard. Campro Scientific was founded in 1981 and we provide services to some of the most prestigious universities, research institutes and laboratories throughout Europe. We are a research-oriented company specialized in supporting the requirements of the scientific community. We are the exclusive distributor of some of the world’s leading producers of research chemicals, radioisotopes, stable isotopes and environmental standards. We understand the requirements of our customers, and work every day to fulfill them. In working with us you are guaranteed to receive: - Excellent customer service - High quality products - Dependable service - Efficient distribution The highly educated staff at Campro’s headquarters and sales office is ready to assist you with your questions and product requirements. Feel free to call us at any time. Sincerely, Dr. Ahmad Rajabi General Manager 180/280 = unlabeled 185/285 = 15N labeled 181/281 = double labeled (13C+15N, 13C+D, 15N+18O etc.) 186/286 = 12C labeled 182/282 = d labeled 187/287 = 17O labeled 183/283 = 13C labeleld 188/288 = 18O labeled 184/284 = 16O labeled, 14N labeled 189/289 = Noble Gases Table of Contents Ordering Information.................................................................................................. page 4 - 5 Packaging Information .............................................................................................. -
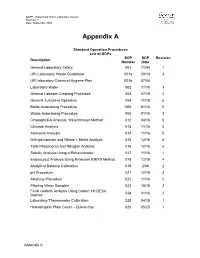
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

1-(4-Amino-Cyclohexyl)
(19) & (11) EP 1 598 339 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 211/04 (2006.01) C07D 211/06 (2006.01) 24.06.2009 Bulletin 2009/26 C07D 235/24 (2006.01) C07D 413/04 (2006.01) C07D 235/26 (2006.01) C07D 401/04 (2006.01) (2006.01) (2006.01) (21) Application number: 05014116.7 C07D 401/06 C07D 403/04 C07D 403/06 (2006.01) A61K 31/44 (2006.01) A61K 31/48 (2006.01) A61K 31/415 (2006.01) (22) Date of filing: 18.04.2002 A61K 31/445 (2006.01) A61P 25/04 (2006.01) (54) 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE DERIVATIVES AND RELATED COMPOUNDS AS NOCICEPTIN ANALOGS AND ORL1 LIGANDS FOR THE TREATMENT OF PAIN 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ON DERIVATE UND VERWANDTE VERBINDUNGEN ALS NOCICEPTIN ANALOGE UND ORL1 LIGANDEN ZUR BEHANDLUNG VON SCHMERZ DERIVÉS DE LA 1-(4-AMINO-CYCLOHEXYL)-1,3-DIHYDRO-2H-BENZIMIDAZOLE-2-ONE ET COMPOSÉS SIMILAIRES POUR L’UTILISATION COMME ANALOGUES DU NOCICEPTIN ET LIGANDES DU ORL1 POUR LE TRAITEMENT DE LA DOULEUR (84) Designated Contracting States: • Victory, Sam AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Oak Ridge, NC 27310 (US) MC NL PT SE TR • Whitehead, John Designated Extension States: Newtown, PA 18940 (US) AL LT LV MK RO SI (74) Representative: Maiwald, Walter (30) Priority: 18.04.2001 US 284666 P Maiwald Patentanwalts GmbH 18.04.2001 US 284667 P Elisenhof 18.04.2001 US 284668 P Elisenstrasse 3 18.04.2001 US 284669 P 80335 München (DE) (43) Date of publication of application: (56) References cited: 23.11.2005 Bulletin 2005/47 EP-A- 0 636 614 EP-A- 0 990 653 EP-A- 1 142 587 WO-A-00/06545 (62) Document number(s) of the earlier application(s) in WO-A-00/08013 WO-A-01/05770 accordance with Art. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Hypothalamic Principles Necessary for the Release of Ovulating Hormone from the Adenohypophysis
EFFECT OF AMINOGLUTETHIMIDE ON REPRODUCTIVE PROCESSES IN FEMALE RATS W. J. EVERSOLE and D. J. THOMPSON Department of Life Sciences, Indiana State University, Terre Haute, Indiana 47809, U.S.A. (Received "òlst January 1974) Summary. Adult female rats injected subcutaneously with 100 mg aminoglutethimide phosphate (AGP)/kg/day for 4 weeks failed to become pregnant when placed in cohabitation with males during the last 2 weeks of the injection period. Lower doses depressed the fertility rate and reduced the litter size: the average litter size of three of eleven rats given 50 mg/kg/day was 4\m=.\7 young and of four of eleven rats given 25 mg was 7\m=.\0 young. Twenty-nine controls averaged 10\m=.\1/litter. Doses of 100 mg AGP/kg/day stopped vaginal cycling, prevented ovulation in adults, and delayed dissolution of the vaginal membrane in pubertal rats. Histological studies of the ovaries from rats with initial ages of 17 or 21 days which were injected with 25 to 100 mg AGP/kg/day for 2 weeks showed an increase in the number and size of vesicular follicles. Treatment of 27-day-old rats with 25 mg/kg/day for 2 weeks reduced the number of CL and three of four rats given 50 mg had ovaries lacking such bodies; all control ovaries in this group contained CL. These findings are taken as evidence that AGP inhibits ovulation but does not prevent follicular maturation. INTRODUCTION Many pharmacological compounds originally developed as anaesthetics, tranquillizers and anticonvulsants modify endocrine structures and functions (see Gaunt, Chart & Renzi, 1965). -

Mechanisms of Action of Antiepileptic Drugs
Review Mechanisms of action of antiepileptic drugs Epilepsy affects up to 1% of the general population and causes substantial disability. The management of seizures in patients with epilepsy relies heavily on antiepileptic drugs (AEDs). Phenobarbital, phenytoin, carbamazepine and valproic acid have been the primary medications used to treat epilepsy for several decades. Since 1993 several AEDs have been approved by the US FDA for use in epilepsy. The choice of the AED is based primarily on the seizure type, spectrum of clinical activity, side effect profile and patient characteristics such as age, comorbidities and concurrent medical treatments. Those AEDs with broad- spectrum activity are often found to exert an action at more than one molecular target. This article will review the proposed mechanisms of action of marketed AEDs in the US and discuss the future of AEDs in development. 1 KEYWORDS: AEDs anticonvulsant drugs antiepileptic drugs epilepsy Aaron M Cook mechanism of action seizures & Meriem K Bensalem-Owen† The therapeutic armamentarium for the treat- patients with refractory seizures. The aim of this 1UK HealthCare, 800 Rose St. H-109, ment of seizures has broadened significantly article is to discuss the past, present and future of Lexington, KY 40536-0293, USA †Author for correspondence: over the past decade [1]. Many of the newer AED pharmacology and mechanisms of action. College of Medicine, Department of anti epileptic drugs (AEDs) have clinical advan- Neurology, University of Kentucky, 800 Rose Street, Room L-455, tages over older, so-called ‘first-generation’ First-generation AEDs Lexington, KY 40536, USA AEDs in that they are more predictable in their Broadly, the mechanisms of action of AEDs can Tel.: +1 859 323 0229 Fax: +1 859 323 5943 dose–response profile and typically are associ- be categorized by their effects on the neuronal [email protected] ated with less drug–drug interactions. -

(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2017 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2017 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Note: Chemicals may be added to or deleted from the list. The Emergency Planning and Community Right-to-Know Call Center or the TRI-Listed Chemicals website will provide up-to-date information on the status of these changes. See section B.3.c of the instructions for more information on the de minimis % limits listed below. There are no de minimis levels for PBT chemicals since the de minimis exemption is not available for these chemicals (an asterisk appears where a de minimis limit would otherwise appear in Table II). However, for purposes of the supplier notification requirement only, such limits are provided in Appendix C. Chemical Qualifiers Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. An EPCRA Section 313 chemical that is listed without a qualifier is subject to reporting in all forms in which it is manufactured, processed, and otherwise used. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical/ Chemical Category CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. -

Chapter 231. Poisons and Dangerous Substances Act 1952
Chapter 231. Poisons and Dangerous Substances Act 1952. Certified on: / /20 . INDEPENDENT STATE OF PAPUA NEW GUINEA. Chapter 231. Poisons and Dangerous Substances Act 1952. ARRANGEMENT OF SECTIONS. PART I – PRELIMINARY. 1. Interpretation. “automatic machine” “the British Pharmacopoeia” “container” “dangerous substance” “methylated spirit” “package” “poison” “poisons licence” “Poisons Register” “rectified spirit” “the regulations” “sell” “this Act” 2. Application. 3. Calculations of percentages. 4. Variation of schedules. PART II – LICENCES. 5. Poisons licence. 6. Revocation of poisons licences. PART III – SALE OF POISONS AND DANGEROUS SUBSTANCES. 7. Application of Part III to wholesalers. 8. Sale of things specified in Schedules 1 and 2. 9. Sale, etc., of things specified in Schedule 1. 10. Sale of things specified in Schedule 3. 11. Sale of things specified in Schedule 4. 12. Sale of things specified in Schedule 5. 13. Hawking poisons and dangerous substances. 14. Sale of poisons to persons under 18 years, etc. 15. Automatic vending machines. 16. Poisons Registers. 17. Entries in Poisons Registers. 18. Person unable to sign his name. 19. Sale on order by letter, etc. 20. Sale to medical practitioners, etc. 21. Application of Sections 14, 17, 18 and 19. 22. Records to be kept by medical practitioners, etc. PART IV – LABELLING AND PACKING POISONS AND DANGEROUS SUBSTANCES. 23. Sale of poisons and dangerous substances. 24. Sale of liquid dangerous substances. 25. Colouring of methylated spirits. 26. Sale of poisons. PART V – MISCELLANEOUS. 27. Drinking of methylated spirit prohibited. 28. Methylated spirit not to be sold for drinking purposes. 29. Inspection. 30. Offences in relation to the sale of poisons, etc. -

Förordning Om Ändring I Förordningen (1992:1554) Om Kontroll Av Narkotika;
Príloha 11 k rozhodnutiu švédskych úradov vlády 22. februára 2018 § 79 1. ------IND- 2018 0079 S-- SK- ------ 20180302 --- --- PROJET Zbierka zákonov Švédska SFS Published on issued on 1 March 2018. The government hereby lays down1 that Annex 1 to the Ordinance (1992:1554) on the control of narcotic drugs2 shall read as set out below. This ordinance shall enter into force on 10 April 2018. On behalf of the Government ANNIKA STRANDHÄLL Lars Hedengran (Ministry of Health and Social Affairs) 1 See Directive (EU) 2015/1535 of the European Parliament and of the Council of 9 September 2015 laying down a procedure for the provision of information in the field of technical regulations and of rules on Information Society services. 2 Ordinance reprinted as 1993:784. 1 SFS Annex 13 List of substances to be considered narcotic drugs according to the Narcotic Drugs Punishments Act Stimulants of the central nervous system ethylamphetamine (2-ethylamino-1-phenylpropane) fenethylline [1-phenyl-1-piperidyl-(2)-methyl]acetate 1-phenyl-2-butylamine N-hydroxyamphetamine propylhexedrine 4-methylthioamphetamine (4-MTA) modafinil 4-methoxy-N-methylamphetamine (PMMA, 4-MMA) 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2) 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) 4-iodo-2,5-dimethoxyphenethylamine (2C-I) 2,4,5-trimethoxyamphetamine (TMA-2) 4-methylmethcathinone (mephedrone) 4-fluoramphetamine 1-(4-methoxyphenyl)-2-(methylamino)propan-1-one (methedrone) 1-(1,3-benzodioxol-5-yl)-2-pyrrolidin-1-yl-pentan-1-one (MDPV) 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butan-1-one