Enzymatic and Histopathologic Biomarkers As Indicators of Aquatic Pollution in Fishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 4 Logistics-Related Facilities and Operation: Land Transport
Chapter 4 Logistics-related Facilities and Operation: Land Transport THE STUDY ON MULTIMODAL TRANSPORT AND LOGISTICS SYSTEM OF THE EASTERN MEDITERRANEAN REGION AND MASTER PLAN FINAL REPORT Chapter 4 Logistics-related Facilities and Operation: Land Transport 4.1 Introduction This chapter explores the current conditions of land transportation modes and facilities. Transport modes including roads, railways, and inland waterways in Egypt are assessed, focusing on their roles in the logistics system. Inland transport facilities including dry ports (facilities adopted primarily to decongest sea ports from containers) and to less extent, border crossing ports, are also investigated based on the data available. In order to enhance the logistics system, the role of private stakeholders and the main governmental organizations whose functions have impact on logistics are considered. Finally, the bottlenecks are identified and countermeasures are recommended to realize an efficient logistics system. 4.1.1 Current Trend of Different Transport Modes Sharing The trends and developments shaping the freight transport industry have great impact on the assigned freight volumes carried on the different inland transport modes. A trend that can be commonly observed in several countries around the world is the continuous increase in the share of road freight transport rather than other modes. Such a trend creates tremendous pressure on the road network. Japan for instance faces a situation where road freight’s share is increasing while the share of the other -

Mints – MISR NATIONAL TRANSPORT STUDY
No. TRANSPORT PLANNING AUTHORITY MINISTRY OF TRANSPORT THE ARAB REPUBLIC OF EGYPT MiNTS – MISR NATIONAL TRANSPORT STUDY THE COMPREHENSIVE STUDY ON THE MASTER PLAN FOR NATIONWIDE TRANSPORT SYSTEM IN THE ARAB REPUBLIC OF EGYPT FINAL REPORT TECHNICAL REPORT 11 TRANSPORT SURVEY FINDINGS March 2012 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS CO., LTD. ALMEC CORPORATION EID KATAHIRA & ENGINEERS INTERNATIONAL JR - 12 039 No. TRANSPORT PLANNING AUTHORITY MINISTRY OF TRANSPORT THE ARAB REPUBLIC OF EGYPT MiNTS – MISR NATIONAL TRANSPORT STUDY THE COMPREHENSIVE STUDY ON THE MASTER PLAN FOR NATIONWIDE TRANSPORT SYSTEM IN THE ARAB REPUBLIC OF EGYPT FINAL REPORT TECHNICAL REPORT 11 TRANSPORT SURVEY FINDINGS March 2012 JAPAN INTERNATIONAL COOPERATION AGENCY ORIENTAL CONSULTANTS CO., LTD. ALMEC CORPORATION EID KATAHIRA & ENGINEERS INTERNATIONAL JR - 12 039 USD1.00 = EGP5.96 USD1.00 = JPY77.91 (Exchange rate of January 2012) MiNTS: Misr National Transport Study Technical Report 11 TABLE OF CONTENTS Item Page CHAPTER 1: INTRODUCTION..........................................................................................................................1-1 1.1 BACKGROUND...................................................................................................................................1-1 1.2 THE MINTS FRAMEWORK ................................................................................................................1-1 1.2.1 Study Scope and Objectives .........................................................................................................1-1 -

Flood-Induced Scour in the Nile by Modified Operation of High Aswan Dam
Conference Paper, Published Version Sloff, C. J.; El-Desouky, I. A. Flood-induced scour in the Nile by modified operation of High Aswan Dam Verfügbar unter/Available at: https://hdl.handle.net/20.500.11970/100075 Vorgeschlagene Zitierweise/Suggested citation: Sloff, C. J.; El-Desouky, I. A. (2006): Flood-induced scour in the Nile by modified operation of High Aswan Dam. In: Verheij, H.J.; Hoffmans, Gijs J. (Hg.): Proceedings 3rd International Conference on Scour and Erosion (ICSE-3). November 1-3, 2006, Amsterdam, The Netherlands. Gouda (NL): CURNET. S. 614-621. Standardnutzungsbedingungen/Terms of Use: Die Dokumente in HENRY stehen unter der Creative Commons Lizenz CC BY 4.0, sofern keine abweichenden Nutzungsbedingungen getroffen wurden. Damit ist sowohl die kommerzielle Nutzung als auch das Teilen, die Weiterbearbeitung und Speicherung erlaubt. Das Verwenden und das Bearbeiten stehen unter der Bedingung der Namensnennung. Im Einzelfall kann eine restriktivere Lizenz gelten; dann gelten abweichend von den obigen Nutzungsbedingungen die in der dort genannten Lizenz gewährten Nutzungsrechte. Documents in HENRY are made available under the Creative Commons License CC BY 4.0, if no other license is applicable. Under CC BY 4.0 commercial use and sharing, remixing, transforming, and building upon the material of the work is permitted. In some cases a different, more restrictive license may apply; if applicable the terms of the restrictive license will be binding. Flood-induced scour in the Nile by modified operation of High Aswan Dam C.J. Sloff* and I.A. El-Desouky ** * WL | Delft Hydraulics and Delft University of Technology, Delft, Netherlands ** Hydraulics Research Institute (HRI), Delta Barrage, Cairo, Egypt Due to climate-change it is anticipated that the hydrological m. -

Water & Waste-Water Equipment & Works
Water & Waste-Water Equipment & Works Sector - Q4 2018 Report Water & Waste-Water Equipment & Works 4 (2018) Report American Chamber of Commerce in Egypt - Business Information Center 1 of 15 Water & Waste-Water Equipment & Works Sector - Q4 2018 Report Special Remarks The Water & Waste-Water Equipment & Works Q4 2018 report provides a comprehensive overview of the Water & List of sub-sectors Waste-Water Equipment & Works sector with focus on top tenders, big projects and important news. Irrigation & Drainage Canals Irrigation & Drainage Networks Tenders Section Irrigation & Drainage Pumping Stations Potable Water & Waste-Water Pipelines - Integrated Jobs (Having a certain engineering component) - sorted by Potable Water & Waste-Water Pumps - Generating Sector (the sector of the client who issued the tender and who would pay for the goods & services ordered) Water Desalination Stations - Client Water Wells Drilling - Supply Jobs - Generating Sector - Client Non-Tenders Section - Business News - Projects Awards - Projects in Pre-Tendering Phase - Privatization and Investments - Published Co. Performance - Loans & Grants - Fairs and Exhibitions This report includes tenders with bid bond greater than L.E. 10,000 and valuable tenders without bid bond Tenders may be posted under more than one sub-sector Copyright Notice Copyright ©2018, American Chamber of Commerce in Egypt (AmCham). All rights reserved. Neither the content of the Tenders Alert Service (TAS) nor any part of it may be reproduced, sorted in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the American Chamber of Commerce in Egypt. In no event shall AmCham be liable for any special, indirect or consequential damages or any damages whatsoever resulting from loss of use, data or profits. -

Food Safety Inspection in Egypt Institutional, Operational, and Strategy Report
FOOD SAFETY INSPECTION IN EGYPT INSTITUTIONAL, OPERATIONAL, AND STRATEGY REPORT April 28, 2008 This publication was produced for review by the United States Agency for International Development. It was prepared by Cameron Smoak and Rachid Benjelloun in collaboration with the Inspection Working Group. FOOD SAFETY INSPECTION IN EGYPT INSTITUTIONAL, OPERATIONAL, AND STRATEGY REPORT TECHNICAL ASSISTANCE FOR POLICY REFORM II CONTRACT NUMBER: 263-C-00-05-00063-00 BEARINGPOINT, INC. USAID/EGYPT POLICY AND PRIVATE SECTOR OFFICE APRIL 28, 2008 AUTHORS: CAMERON SMOAK RACHID BENJELLOUN INSPECTION WORKING GROUP ABDEL AZIM ABDEL-RAZEK IBRAHIM ROUSHDY RAGHEB HOZAIN HASSAN SHAFIK KAMEL DARWISH AFKAR HUSSAIN DISCLAIMER: The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. CONTENTS EXECUTIVE SUMMARY...................................................................................... 1 INSTITUTIONAL FRAMEWORK ......................................................................... 3 Vision 3 Mission ................................................................................................................... 3 Objectives .............................................................................................................. 3 Legal framework..................................................................................................... 3 Functions............................................................................................................... -

World Bank Document
PROCUREMENT PLAN (Textual Part) Project information: Egypt Transforming Egypt's Healthcare System Project P167000 Project Implementation agency: Ministry of Health and Population Public Disclosure Authorized Date of the Procurement Plan: October 23, 2018 Period covered by this Procurement Plan: 18 months Preamble In accordance with paragraph 5.9 of the “World Bank Procurement Regulations for IPF Borrowers” (July 2016) (“Procurement Regulations”) the Bank’s Systematic Tracking and Exchanges in Procurement (STEP) system will be used to prepare, clear and update Procurement Plans and conduct all procurement transactions for the Project. Public Disclosure Authorized This textual part along with the Procurement Plan tables in STEP constitute the Procurement Plan for the Project. The following conditions apply to all procurement activities in the Procurement Plan. The other elements of the Procurement Plan as required under paragraph 4.4 of the Procurement Regulations are set forth in STEP. The Bank’s Standard Procurement Documents: shall be used for all contracts subject to international competitive procurement and those contracts as specified in the Procurement Plan tables in STEP. Public Disclosure Authorized National Procurement Arrangements: In accordance with paragraph 5.3 of the Procurement Regulations, when approaching the national market (as specified in the Procurement Plan tables in STEP), the country’s own procurement procedures may be used. Leased Assets: “Not Applicable” Procurement of Second Hand Goods: “Not Applicable” Domestic -
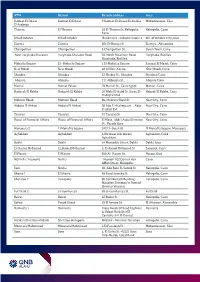
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St. -

Geographic Distribution of Inflammatory Breast Cancer and Non-Inflammatory Breast Cancer in Gharbiah, Egypt an Nguyen1, Amr S
Nguyen A, Soliman AS, Hablas A, Ramadan M, Seifeldin I. Geographic Distribution of Journal of Inflammatory Breast Cancer and Non-Inflammatory Breast Cancer in Gharbiah, Egypt. J Rare Diseases Research Rare Dis Res Treat. (2020) 5(2): 6-12 & Treatment www.rarediseasesjournal.com Research Article Open Access Geographic Distribution of Inflammatory Breast Cancer and Non-Inflammatory Breast Cancer in Gharbiah, Egypt An Nguyen1, Amr S. Soliman2*, Ahmed Hablas3, Mohamed Ramadan3, Ibrahim Seifeldin3 1Milken Institute School of Public Health, The George Washington University, Washington, DC, USA 2CUNY School of Medicine, New York, NY, USA 3Gharbiah Cancer Society, Tanta, Gharbiah, Egypt Article Info ABSTRACT Article Notes BACKGROUND: Inflammatory Breast Cancer (IBC) is a rare but aggressive Received: July 01, 2020 form of breast cancer, constituting about 1-5% of breast cancers in the U.S. Accepted: July 23, 2020 but about 11% in Egypt. The etiology of IBC is unknown, but the distribution *Correspondence: of residence of IBC and non-IBC patients may provide clues to the disease risk Dr. Amr S. Soliman, City University of New York School of factors. Therefore, we investigated the geographic distribution of IBC and non- Medicine, New York, NY, USA; Telephone No: +1(212) 650- IBC in the province of Gharbiah, Egypt. 8519; Fax: +1(212) 650-7778; Email: [email protected] METHODS: All breast cancers diagnosed during the period of January 2009 to December 2010 were retrieved from the Gharbiah Population-based © 2020 Soliman AS. This article is distributed under the terms of registry. IBC cases were obtained from a case-control study conducted in the the Creative Commons Attribution 4.0 International License. -

5 Environmental and Social Impacts ______27 5.1 Introduction ______Error! Bookmark Not Defined
Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Submitted to : Egyptian Natural Gas Holding Company EGAS ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT FRAMEWORK Prepared by: Executive Summary Public Disclosure Authorized NATURAL GAS CONNECTION PROJECT IN 20 GOVERNORATES IN EGYPT (January 2017) Executive summary 1 List of acronyms and abbreviations AFD Agence Française de Développement (French Agency for Development) BUTAGASCO The Egyptian Company for LPG distribution CAPMAS Central Agency for Public Mobilization and Statistics EHDR Egyptian Human Development Report 2010 EEAA Egyptian Environmental Affairs Agency EGAS Egyptian Natural Gas Holding Company EGP Egyptian pound ESDV Emergency Shut Down Valve ESIAF Environmental and Social Impact Assessment Framework ESMMF Environmental and Social Management and Monitoring Framework ESMP Environmental and Social Management Plan FGD Focus Group Discussion GoE Government of Egypt HP High Pressure HSE Health Safety and Environment LDC Local Distribution Companies LPG Liquefied Petroleum Gas LP Low Pressure mBar milliBar NG Natural Gas NGO Non-Governmental Organizations PAP Project Affected Persons PRS Pressure Reduction Station QRA Quantitative Risk Assessment RAP Resettlement Action Plan RPF Resettlement Policy Framework SDO Social Development Officer SFD Social Fund for Development SSIAF Supplementary Social Impact Assessment Framework TOR Terms of Reference Town Gas The Egyptian Company for Natural Gas Distribution for Cities WB The World Bank US $ United -

1.5 Million Natural Gas Connections Project in 11 Governorates Zefta High Pressure Pipeline /Gharbia Governorate Due Diligence
– 1.5 Million Natural Gas Connections Project in 11 Governorates Zefta High Pressure Pipeline /Gharbia Governorate Due Diligence EGAS Egyptian Natural Gas Holding Company Final report December 2019 Developed by EcoConServ Environmental Solutions 1.5 Million Natural Gas Connections Project in 11 Governorates, Due Diligence, Zefta PRS /Gharbia Governorate Final Report, November 2019 Contents CONTENTS _______________________________________________________________ I LIST OF ANNEXES ________________________________________________________ II LIST OF TABLES ___________________________________________________________ II LIST OF FIGURES ________________________________________________________ III LIST OF ACRONYMS AND ABBREVIATIONS _________________________________ IV EXECUTIVE SUMMARY ____________________________________________________ I 1 INTRODUCTION ___________________________________________________ 18 1.1 PREAMBLE ____________________________________________________ 18 1.2 DUE DILIGENCE OBJECTIVES AND METHODOLOGY ____________________ 19 1.3 CONTRIBUTORS ________________________________________________ 20 1.4 DESCRIPTION OF THE PROJECT AREAS _______________________________ 20 1.5 PROJECT WORK PACKAGES _______________________________________ 22 1.6 PROJECT ACTIVITIES OF CONSTRUCTION PHASE _______________________ 23 1.7 ACTIVITIES OF OPERATIONAL PHASE ________________________________ 25 1.8 RESOURCES CONSUMPTION _______________________________________ 26 1.9 WASTE GENERATION ____________________________________________ 26 1.10 AVOIDANCE MECHANISM -

Nile Delta Drainage Project - Workshops Engineer's Final Report - March 10, 1972
THE WORLD BANK GROUP ARCHIVES PUBLIC DISCLOSURE AUTHORIZED Folder Title: Arab Republic of Egypt Nile Delta Authority For Tile Drainage Projects - Nile Delta Drainage Project - Workshops Engineer's Final Report - March 10, 1972 Folder ID: 274351 Project ID: P004983 Fonds: Records of the Middle East and North Africa Regional Vice Presidency ISAD Reference Code: WB IBRD/IDA MNA Digitized: 9/24/2018 To cite materials from this archival folder, please follow the following format: [Descriptive name of item], [Folder Title], Folder ID [Folder ID], World Bank Group Archives, Washington, D.C., United States. The records in this folder were created or received by The World Bank in the course of its business. The records that were created by the staff of The World Bank are subject to the Bank's copyright. Please refer to http://www.worldbank.org/terms-of-use-earchives for full copyright terms of use and disclaimers. M THE WORLD BANK Washington, D.C. @ International Bank for Reconstruction and Development / International Development Association or The World Bank 1818 H Street NW Washington DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org NILE DELTA AUTHORITY FOR TILE DRAINAGE PROJECTS NILE DEITA DRAINAGE PROJECT Workshops Engineer'sa F:inal Report scri' 10 March, 1972 Stockolm, wede ~VATT _GNDSYA conultngEngners ndArhiecot, Table of Contents 1. INTRODUCTION 1.1 The Workshops Engineer and his cooperation with the Authority 1.2 Brief description of the Workshops Engineer's duties 1 .3 General about the Project and the IDA Report 2. BRIEF DESCRIPTION OF THE WORKSHOPS ENGINEER'S WORK 3. PROJECT EQUIPMENT TO BE REPAIRED IN THE AUTHORITY'S WORKSHOPS 3.1 The amount of construction and transport equipment for the Project as estimated in the IDA Report 3.2 Construction equipment 3.3 Tractors, trailers and trucks for the transport of heavy equipment and materials 3.4 The Authority's vehicles and various other equipment 4. -

Effect of Polluted Water on Soil, Sediments and Plant Contamination
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Solid Earth Discuss., doi:10.5194/se-2015-34, 2016 Manuscript under review for journal Solid Earth Published: 8 February 2016 SED © Author(s) 2016. CC-BY 3.0 License. doi:10.5194/se-2015-34 This discussion paper is/has been under review for the journal Solid Earth (SE). Effect of polluted Please refer to the corresponding final paper in SE if available. water on soil, sediments and plant Effect of polluted water on soil, sediments contamination and plant contamination by heavy metals E. Mahmoud and A. M. Ghoneim in El-Mahla El-Kobra, Egypt Title Page E. Mahmoud1 and A. M. Ghoneim2,3 Abstract Introduction 1Soil and Water Sciences Dept., Faculty of Agriculture at Tanta, Tanta University, Tanta, Egypt 2Soil Science Department, College of Food and Agricultural Sciences, King Saud University, Conclusions References P.O. Box 2460, Riyadh 11451, Saudi Arabia Tables Figures 3Agricultural Research Center, Field Crops Research Institute, Rice Research and Training Center, Sakha, 33717, Kafr El-Sheikh, Egypt J I Received: 24 March 2015 – Accepted: 25 March 2015 – Published: 8 February 2016 J I Correspondence to: E. Mahmoud ([email protected]) Back Close Published by Copernicus Publications on behalf of the European Geosciences Union. Full Screen / Esc Printer-friendly Version Interactive Discussion 1 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract SED The discharge of untreated wastewater in drains Zefta and No. 5 is becoming a problem for many farmers in El-Mahla El-Kobra area, Egypt. The discharging water contains doi:10.5194/se-2015-34 high levels of contaminants considered hazardous to the ecosystem.