Mineralogical Prediction on the Flotation Behavior of Copper and Molybdenum Minerals from Blended Cu–Mo Ores in Seawater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -
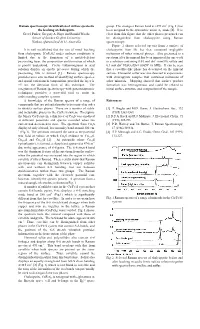
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

Stability of Metallic Copper in the Near Surface Environment
OC ~l CXU ^J'JI. T KJ STATENS KÄRNBRÄNSLE NÄMND NATIONAL BOABD FOR SPENT NUCLEAR FUEL SKN REPORT 57 . Stability of Metallic Copper in the Near Surface Environment MARCH 1992 Where and how will we dispose of spent nuclear fuel? There is political consensus to dispose or spent nuclear fuel from Swedish nuclear power plants in Sweden. No decision has yet been reached on a site for the final repository in Sweden and neither has a method for disposal been determined. The disposal site and method must be selected with regard to safety and the environment as well as with regard to our responsibility to prevent the proliferation of materials which can be used to produce nuclear weapons. In 1983, a disposal method called KBS-3 was presented by the nuclear power utilities, through the Swedish Nuclear Fuel and Waste Management Company (SKB). In its 1984 resolution on permission to load fuel into the Forsmark 3 and Oskarshamn 3 reactors, the government stated that the KBS-3 method - which had been thoroughly reviewed by Swedish and foreign experts - "was, in its entirety and in all essentials, found to be acceptable in terms of safety and radiological protection." In the same resolution, the government also pointed out that a final position on a choice of method would require further research and development work. Who is responsible for the safe management of spent nuclear fuel? The nuclear power utilities have the direct responsibility for the safe handling and disposal of spent nuclear fuel. This decision is based on the following, general argument: those who conduct an activity arc responsible for seeing that the activity is conducted in a safe manner. -

New Data on the Hyrkkola Native Copper Mineralization: a Natural Analogue for the Long-Term Corrosion of Copper Canisters
NEW DATA ON THE HYRKKOLA NATIVE COPPER MINERALIZATION: A NATURAL ANALOGUE FOR THE LONG-TERM CORROSION OF COPPER CANISTERS N. MARCOS’, L. AHONEN’, R. BROS’, P. ROOS4, J. SUKS15 & V. OVERSBY6 ’ Helsinki University of Technology, Engineering Geology & GeoDhvsics Lab. I< .- P.O. Box 6200, FiN-02015 HUT Finknd Geoloeical Survev of Finland (GTK). P.O. Box 96. FIN-02151. Esooo. Finland ’ Envir&mental Geochemistry Laboratory, Department of EnvironmeAtal Safety Research, JAERI, Tokai, Ibaraki, 319-1 1 Japan University of Lund, 22185 Lund, Sweden Laboratory of Radiochemistry, P.O. Box 55 FIN-00014, Helsinki, Finland VMO Konsult, Stockholm, Sweden ABSTRACT The Hyrkkola U-Cu mineralization located in south-western Finland is reassessed with reference to the corrosion mechanisms affecting the stability of native copper and the time-scales of corrosion processes. The mineral assemblage native copper - copper sulfide occurs in open fractures at several depth intervals within granite pegmatites (GP). The surfaces of these open fractures have accumulations of uranophane crystals and other unidentified uranyl compounds. The secondary uranium minerals are mainly distributed around copper sulfide grains. Microscopic intergrowths of copper sulfides and uranyl compounds also have been observed. Groundwater samples were collected from the vicinity of the Cu samples. The hydrogeochemical features of these samples indicate that the present conditions are oxidising. The minimum age of U(V1) transport and deposition is about 200 000 years. This age is indicated by 234U/238Uand 230Th/234Uactivity ratios of uranophane. The age of the hexavalent uranium precipitation may be somewhat later than the last influxes and/or demobilisation of sulfur. The mineral assemblage native copper - copper oxide (cuprite) occurs only at one depth interval within altered granite pegmatite. -

AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
THE AMERICAN MINERALOGIST, VOL. 55, MAY_JUNE, 1970 AN OCCURRENCE OF THE ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.s"",AUCANQUILCHA, CHILE AraN H. Cr-etr<, Deportmentof GeologicalSciences, Queen's U niaersity, Kingston, Ontario. AssrnA.cr A mineral with composition near to CusFeSo has been found associated with nzrtive sulfur and covellite in the volcanic sulfur deposit at Aucanquilcha. Microprobe, optical and X-ray powder diffraction data match closell'a previously reported occurrence at Nu- kundamu, Fiji. Comparison with equilibrium synthesis by Kullerud and others indir:ates formation in the temperature range 434-482"C. INrnonucrroN Electron probe microanalysis(L6vy, 1967; Sillitoe and Clark, lt69) of naturally-occurring,supergene idaite has cast considerabledoub1. on the equation (Frenzel, 1959) of this not uncommon sulfi.de with the phaseof generalformula Cur r,Fe,Se.s,1 (Yund, 1963), which has h,een synthesizedby Merwin and Lombard (1937), Roseboomand Kullerud (1958),and Yund and Kullerud (1966).Idaite has been shown to have the compositionCurFeSa, or Cu3FeS4-,1orrd there is as yet no evidence of solid solution in nature between this and more copper-rich comF,osi- tions.The well-establishedX-ray powder data"(a:3.772 A; c:11.1U A; Yund, 1963) for hexagonal Cus.s,Fe"Se.r, are only with difficulty recon- ciled with thoseof natural idaite (Frenzel,1959, 1963), and L6vy (I\167) has suggestedthat Frenzel'soriginal powder data may be adequately fitted to a stannite-type,tetragonal cell. A differencein crystal struclure between thesephases is supported by the dissimilar reflectivity disper- sion profi.lesof natural idaite (L6vy, 1967; Sillitoe and Clark, 1969) and the synthetic "Cu5FeS6"of Merwin and l-ombard (1937; inLlvy, 1967). -

Michigan's Copper Country" Lets You Experience the Require the Efforts of Many People with Different Excitement of the Discovery and Development of the Backgrounds
Michigan’s Copper Country Ellis W. Courter Contribution to Michigan Geology 92 01 Table of Contents Preface .................................................................................................................. 2 The Keweenaw Peninsula ........................................................................................... 3 The Primitive Miners ................................................................................................. 6 Europeans Come to the Copper Country ....................................................................... 12 The Legend of the Ontonagon Copper Boulder ............................................................... 18 The Copper Rush .................................................................................................... 22 The Pioneer Mining Companies................................................................................... 33 The Portage Lake District ......................................................................................... 44 Civil War Times ...................................................................................................... 51 The Beginning of the Calumet and Hecla ...................................................................... 59 Along the Way to Maturity......................................................................................... 68 Down the South Range ............................................................................................. 80 West of the Ontonagon............................................................................................ -

Copper Deposits in Sedimentary and Volcanogenic Rocks
Copper Deposits in Sedimentary and Volcanogenic Rocks GEOLOGICAL SURVEY PROFESSIONAL PAPER 907-C COVER PHOTOGRAPHS 1 . Asbestos ore 8. Aluminum ore, bauxite, Georgia 1 2 3 4 2. Lead ore. Balmat mine, N . Y. 9. Native copper ore, Keweenawan 5 6 3. Chromite-chromium ore, Washington Peninsula, Mich. 4. Zinc ore, Friedensville, Pa. 10. Porphyry molybdenum ore, Colorado 7 8 5. Banded iron-formation, Palmer, 11. Zinc ore, Edwards, N.Y. Mich. 12. Manganese nodules, ocean floor 9 10 6. Ribbon asbestos ore, Quebec, Canada 13. Botryoidal fluorite ore, 11 12 13 14 7. Manganese ore, banded Poncha Springs, Colo. rhodochrosite 14. Tungsten ore, North Carolina Copper Deposits in Sedimentary and Volcanogenic Rocks By ELIZABETH B. TOURTELOT and JAMES D. VINE GEOLOGY AND RESOURCES OF COPPER DEPOSITS GEOLOGICAL SURVEY PROFESSIONAL PAPER 907-C A geologic appraisal of low-temperature copper deposits formed by syngenetic, diagenetic, and epigenetic processes UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director First printing 1976 Second printing 1976 Library of Congress Cataloging in Publication Data Tourtelot, Elizabeth B. Copper deposits in sedimentary and volcanogenic rocks. (Geology and resources of copper) (Geological Survey Professional Paper 907-C) Bibliography: p. Supt. of Docs. no.: I 19.16:907-C 1. Copper ores. 2. Rocks, Sedimentary. 3. Rocks, Igneous. I. Vine, James David, 1921- joint author. II. Title. III. Series. IV. Series: United States Geological Survey Professional Paper 907-C. TN440.T68 553'.43 76-608039 For sale by the Superintendent of Documents, U.S. -

Covellite) Probed by NQR
Phase transition and anomalous electronic behavior in layered dichalcogenide CuS (covellite) probed by NQR R.R. Gainov 1*, A.V. Dooglav 1, I.N. Pen’kov 2, I.R. Mukhamedshin 1,3, N.N. Mozgova 4, A.V. Evlampiev 1, and I.A. Bryzgalov 5 1 Department of Physics, Magnetic RadioSpectroscopy Laboratory, Kazan State University, Kazan, Kremlevskaya str. 18, 420008, Russian Federation 2 Department of Geology, Kazan State University, Kazan, Kremlevskaya str. 4/5, 420111, Russian Federation 3 Laboratoire de Physique des Solides, UMR 8502, Universite Paris-Sud, 91405 Orsay, France 4 Institute of geology of ore deposits, petrography, mineralogy and geochemistry (Russian Academy of Science), Staromonetny per. 35, Moscow 109017, Russian Federation 5 Department of Geology, Moscow State University, Moscow, Vorob’evy gory, 119991, Russian Federation * Corresponding author: tel.: 7-843-2315175; fax.: 7-843-2387201. Electronic address: [email protected] (R.R. Gainov). Nuclear quadrupole resonance (NQR) on copper nuclei has been applied for studies of the electronic properties of quasi-two-dimensional low-temperature superconductor CuS (covellite) in the temperature region between 1.47 and 290 K. Two NQR signals corresponding to two non- equivalent sites of copper in the structure, Cu(1) and Cu(2), has been found. The temperature dependences of copper quadrupole frequencies, line-widths and spin-lattice relaxation rates, which so far had never been investigated so precisely for this material, altogether demonstrate the structural phase transition near 55 K, which accompanies transformations of electronic spectrum not typical for simple metals. The analysis of NQR results and their comparison with literature data show that the valence of copper ions at both sites is intermediate in character between monovalent and divalent states with the dominant of the former. -

149. Crystal Structure of Enargite (Cu3ass4)
524 [Vol. 9, 149. Crystal Structure of Enargite (Cu3AsS4). By Katsutoshi TAKANE. Institute of Mineralogy, Petrology and Economic Geology, Tohoku Imperial University, Sendai. (Rec. Nov. 11, 1933. Comm. by S. Kozu, M.I.A., Nov. 13, 1933.) Recently, the crystal structures of copper sulphides such as covellite (CuS), wolfsbergite (CuSbS2), emplectite (CuBiS2), chalcopyrite (CuFeS2) and sulvanite (Cu3VS4), have been worked out by different authors. Among these minerals, sulvanite has been grouped in the mineral family to which enargite belongs, because of the similarity in their chemical compositions. However they are different in crystallographic nature, as sulvanite belongs the cubic system of the space group T1d, determined by Pauling and Hultgren, and enargite belongs to the orthor hombic system of the space group V12h,determined by the present author. Symmetry:-According to the morphological studies already made, enargite belongs to the orthorhombic holodedral class, the axial ratio being given as a : b : c=0.8694 : 1 : 0.8308 by Groth and Mieleitner. The Laue photograph taken from (001) shows no objection to taking the crystal as possessing the symmetry of the orthorhombic holodedral class. It is noteworthy that the photograph indicates a pseudohexagonal symmetry, of which a brief discussion has already been written in Japanese. Unit cell:-From three reflection photographs taken by rotation of three mineral rods parallel to [001], [010] and [100] respectively, immersing in the beam of the CuK ray, the distances of the layer lines were measured, and the results are The axial ratio obtained from the above figures is a : b : c=1.7341.7 1.674, which are double the values of a and c given by the goniometric method. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

CUPRITE Calcite, Which Impart a Carmine Red Color to That Cu2o Mineral (Yedlin, 1974; Morris, 1983)
in interstices between conglomerate boulders. Also as variety “chalcotrichite” and as inclusions in CUPRITE calcite, which impart a carmine red color to that Cu2O mineral (Yedlin, 1974; Morris, 1983). 2. Clark A secondary copper mineral formed by supergene mine, Copper Harbor. 3. North Cliff mine: Fine alteration of native copper (q.v.) and copper micro-crystals showing gyroidal forms in nodules sulfides. Much of the native copper from the of copper (Moore and Beger, 1963). 4. Cliff mine: Keweenaw has films of cuprite, some of which With azurite as massive nodules cemented by formed after the mines were opened, but some of copper and, locally, silver. A few vugs lined with which, particularly from the higher levels, predates excellent crystals were found by Williams (1966). the mining (Butler and Burbank, 1929). Usually these crystals are faced by {001}, {011}, “Chalcotrichite” is a capillary or fibrous variety. and {111} of about equal development. Some Northern Peninsula. crystals show gyroidal symmetry with {321} left, and, if this is present, {211} is a major form. 5. Houghton County: 1. Centennial mine. 2. Delaware mine (Morris, 1983). 6. In stromatolites Laurium and La Salle mines, Osceola. 3. Quincy (calcite) in the Copper Harbor Conglomerate near mine, Hancock (2, 3 Morris, 1983). Gemmy red Copper Harbor with copper (q.v.), domeykite, and crystals up to 1 mm encrusting copper and quartz copper sulfides (Nishioka et al., 1983). crystals have been found on the 7th level of the Microscopic. 7. At the Central and Phoenix Quincy amygdaloid lode, 50 to 100 meters mines: As a red patina on native copper crystals, northeast of the No. -

Chalcocite Several Centimeters Wide Were Found (Butler and Burbank, 1929)
Veinlets of solid chalcocite several centimeters wide were found (Butler and Burbank, 1929). 2. CHALCOCITE Isle Royale mine: In fissures with “ankerite” and Cu2S “specularite.” Lane (1911) reports veins of Widespread, but historically not significant as an chalcocite, bornite, chalcopyrite, “whitneyite,” ore mineral in Michigan, except at the White Pine pyrrhotite(?), natrolite, analcime, and “adularia.” 3. deposit; in fissure veins cutting various rocks of Centennial mine: Disseminated in the Calumet and the native copper lodes and locally disseminated in Hecla Conglomerate. 4. Champion mine: In the lodes themselves; also present in a variety of veinlets with silver. Some as very fine-grained metalliferous veins. Twelve chalcocite deposits pulverulent material consisting of hexagonal have been located on the Keweenaw Peninsula, the platelets less than 0.5 mm across (Koenig, 1902). most notable being the Mount Bohemia and 5. Portage mine: In fissure veins with quartz and Gratiot Lake deposits (Robertson, 1975; Maki, orange calcite. 6. Wolverine mine. 7. Osceola 1999). Maki (1999) estimates the Gratiot Lake mine: As complex microcrystals and crusts on deposit may contain as much as 4.5 million metric prehnite from the No. 10 shaft (Falster, 1978). 8. tons of ore with an average grade of 2.3% copper. East slope Six Mile Hill, southwest of Houghton: Northern Peninsula. Networks of veins with copper, calcite, epidote, prehnite, datolite, and a considerable amount of chalcocite (Rominger, 1895). 9. Laurium mine: (Morris, 1983). Iron County: Sherwood and Buck iron mines: With other sulfides and uraninite (Vickers, 1956b; James et al., 1968). Figure 54: Chalcocite crystals from the White Pine mine, White Pine, Ontonagon County.