Phase Transition and Surface Sublimation of a Mobile Potts Model a Bailly-Reyre, H.T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -
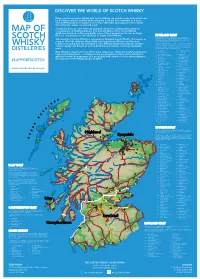
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Hydro-Distillation and Steam Distillation from Aromatic Plants
Hydro-distillation and steam distillation from aromatic plants Sudeep Tandon Scientist Chemical Engineering Division CIMAP, Lucknow HISTORY Written records of herbal distillation are found as early as the first century A.D., and around 1000 A.D., the noted Arab physician and naturalist Ibn Sina also known as Avicenna described the distillation of rose oil from rose petals The ancient Arabian people began to study the chemical properties of essential oils & developed and refined the distillation process Europeans began producing essential oils in the 12th century 1 DISTILLATION ? A process in which a liquid or vapour mixture of two or more substances is separated into its component fractions of desired purity, by the application and removal of heat. In simple terms distillation of aromatic herbs implies vaporizing or liberating the oils from the trichomes / plant cell membranes of the herb in presence of high temperature and moisture and then cooling the vapour mixture to separate out the oil from water. It is the most popular widely used and cost effective method in use today for producing majority of the essential oils throughout the world Distillation is an art and not just a “Chemical" process that is reliant upon many factors for successful quality oil production. BASIC SCIENTIFIC PRINCIPLES INVOLVED IN THE PROCESS To convert any liquid into a vapour we have to apply energy in form of heat called as latent heat of vaporization A liquid always boils at the temperature at which its vapour pressure equals the atmospheric / surrounding pressure For two immiscible liquids the total vapour pressure of the mixture is always equal to the sum of their partial pressures The composition of the mixture will be determined by the concentration of the individual components into its partial pressure As known the boiling point of most essential oil components exceeds that of water and generally lies between 150 – 300oC 2 If a sample of an essential oil having a component ‘A’ having boiling point for example 190oC and the boiling point of the water is 100oC. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Distillation of Essential Oils1
WEC310 Distillation of Essential Oils1 Elise V. Pearlstine 2 A short history of essential oils many industries and in new applications as awareness of the benefit of naturally derived products grows. Essential oils are volatile, aromatic oils obtained from plants and used for fragrance, flavoring, and health and beauty applications. Historically, aromatic plants provided important ingredients for perfumes, incense, and cosmetics. They have also been used for ritual purposes and in cooking and medicine. Egyptians used aromatic plant materials to preserve mummies, the Ayurvedic literature of India includes many references to scented substances, ancient Chinese herbalists valued them for their curative properties, and royalty used rare aromatics to perfume themselves and their surroundings. Distillation became an important An eighteenth century still from an old method of obtaining the healing and fragrant Figure 1. monograph by Gildemeister. components of various plants and was well-studied beginning in the 18th and continuing in the 19th Plant anatomy and structure as they centuries (Figure 1). In the 1900s, during the time of relate to essential oil production the industrial revolution, component parts of many essential oils were identified. These components An essential oil is the volatile material derived could then be synthesized for use in perfume and from plant material by a physical process. The plant flavor industries. The art of using essential oils material is usually aromatic and of a single botanical declined during this time but experienced a re-birth in species and form; some essential oil plants have a Europe with aromatherapy later in the century. In different chemical makeup depending on the variety recent years, the use of essential oils has increased in of plant, and the essential oils are correspondingly unique. -

A Guide to Stills & Distilling
A GUIDE TO STILLS & DISTILLING LEADERS IN HOME DISTILLING AND FILTRATION SYSTEMS THAT MEET AND EXCEED EVEN COMMERCIAL STANDARDS. www.stillspirits.com Reorder: 72648 V1 Reorder: With over 25 years in the Air Still A compact, sleek stainless steel still for those who want a no fuss way market, Still Spirits is a leader of producing 1 L (1 US qt) of 40% ABV spirit at a time. Distils alcohol at 60% strength. Used with a small 10 L (2.6 US Gal) fermenter making this in home distilling and filtration system easy to move and operate from any kitchen, boat or campervan. systems that meet and exceed AVERAGE USE even commercial standards. DIFFICULTY Basic CAPACITY 4 L (1 US Gal) The range features: TIME 7- 10 day Fermentation Premium spirits with equivalent commercial quality Approx 2 hours distilling per 1 L (1 US qt) of 40% alcohol at a fraction of the price. YIELD 2 L (2 US qt) of 40% ABV alcohol per 8 L Homemade spirits in as little as 7 days. (2 US Gal) wash Distilled alcohol from a wash made simply using sugar, PURITY Distils at 60% ABV (120 US proof) before being watered down to 40% (80 US proof) yeast, carbon and drinkable water. Homemade spirit flavoured with your choice of Still Spirits spirit or liqueur essences. QUICK GUIDE TO DISTILLING FEATURES WITH THE AIR STILL 1. Wash – Make an 8 L (2 US Gal) wash by • Easy to operate, set up and use. mixing water, sugar, carbon and yeast With no hoses or complicated in the fermenter. -

Canadian Whisky, American Whiskey & Bourbon – Corn, Rye, Malt
TITLE ADDING BEVERAGE ALCOHOL DISTILLATION TO AN EXISTING BREWERY Presented by Stephen Harkness, P.Eng., President, cemcorp LTD. MBAC District Ontario Spring Technical Meeting, April 23, 2015 HISTORY CANADIAN DISTILLERS 2015 RETAIL STORE POT STILL DIFFERENCES DIFFERENCES BREWERY VS. DISTILLERY 1. NOMENCLATURE/ 5. DISTILLATION TERMINOLOGY PROCESS 2. RAW MATERIAL 6. FINAL PRODUCT 3. MASHING/COOKING 7. BUILDING DESIGN 4. FERMENTATION 8. LICENSING PROCESS REQUIREMENTS NOMENCLATURE – PROOF STRENGTH 1. NOMENCLATURE PROOF STRENGTH U.S. DEFINITION: 1 PROOF = 0.5% A/V i.e. 200 PROOF = 100% A/V BRITISH DEFINITION: 100 PROOF = 57.06% A/V i.e. 175.25 PROOF = 100% A/V METRIC DEFINITION: 1°GL (GAY LUSSAC) = 1% A/V i.e. 100°GL = 100% a/v NOMENCLATURE 1. NOMENCLATURE • BREWERS MEASURE VOLUMES IN HECTOLITRES 1 HL = 0.85 US Barrels = 0.61 UK Barrels (BBL) DISTILLERS MEASURE VOLUMES IN LAA (LITRES ABSOLUTE ALCOHOL) OR PROOF GALLONS • CANADIAN CASE OF BEER IS 24 BOTTLES 341 ml CASE OF LIQUOR IS 12 BOTTLES 750 ml • CANADIAN BREWERS STATE STRENGTH AS %A/V (AMERICAN BREWERS STATE STRENGTH AS %A/W) DISTILLERS STATE STRENGTH AS % OF LAA • REFLUX, DEPHLEGMATOR, DOWNPIPE, WEIR, CONGENERS RAW MATERIAL 2. RAW MATERIAL WHISKY/WHISKEY, including CANADIAN WHISKY, AMERICAN WHISKEY & BOURBON – CORN, RYE, MALT SCOTCH WHISKY AND IRISH WHISKEY – MALT RUM – MOLASSES OR CANE JUICE VODKA – ANY STARCH OR SUGAR GRAPPA, LIQUEURS, BRANDIES SNAPPS - FRUIT MASHING/COOKING 3. MASHING/COOKING 1. BATCH COOKERS 2. CONTINUOUS COLUMNAR COOKERS 3. U-TUBE CONTINUOUS JET COOKERS 4. EXTRUDERS FERMENTATION 4. FERMENTATION BREWERIES FERMENT 5 – 20 DAYS @ 12°C. – 20° C. -

Azeotropes As Powerful Tool for Waste Minimization in Industry and Chemical Processes
molecules Review Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes Federica Valentini and Luigi Vaccaro * Laboratory of Green S.O.C., Dipartimento di Chimica, Biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto 8, 06123 Perugia, Italy; [email protected] * Correspondence: [email protected]; Tel./Fax: +39-07-5585-5541 Academic Editor: Derek J. McPhee Received: 17 October 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: Aiming for more sustainable chemical production requires an urgent shift towards synthetic approaches designed for waste minimization. In this context the use of azeotropes can be an effective tool for “recycling” and minimizing the large volumes of solvents, especially in aqueous mixtures, used. This review discusses the implementation of different kinds of azeotropic mixtures in relation to the environmental and economic benefits linked to their recovery and re-use. Examples of the use of azeotropes playing a role in the process performance and in the purification steps maximizing yields while minimizing waste. Where possible, the advantages reported have been highlighted by using E-factor calculations. Lastly azeotrope potentiality in waste valorization to afford value-added materials is given. Keywords: azeotrope; waste minimization; solvent recovery; azeotropic distillation; process design; waste valorization 1. Introduction The materials not incorporated into the final desired product and the energy required for conducting a process can be considered most trivially as the major direct origin of “waste” associated to chemical production. In addition, the possible formation of undesired isomers or byproducts also results in the generation of costly waste, that being in some cases hazardous, can limit the final application of the main product. -

Fractional and Simple Distillation of Cyclohexane and Toluene from Unknown Sample Z
FRACTIONAL AND SIMPLE DISTILLATION OF CYCLOHEXANE AND TOLUENE FROM UNKNOWN SAMPLE Z Douglas G. Balmer (T.A. Mike Hall) Dr. Dailey Submitted 18 July 2007 Balmer 1 Introduction : The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions using fractional distillation. The two components of the mixture are able to be separated because toluene’s boiling point is 30˚C higher than cyclohexane’s boiling point. Cyclohexane should boil and distil before toluene. Even though their boiling points are different, it is difficult to isolate each component entirely. The vapor above the solution that is formed during distillation is not pure. It is a mixture of cyclohexane and toluene vapors. The composition of that vapor mixture is dependent upon the solution’s composition according to Raoult’s law which states that the partial vapor pressure (P X) of a component at a specific o temperature is equal to the pure vapor pressure (P X) of that component multiplied by the mole fraction (N X) of that component in solution (Eq. 1). o PX = P XNX (1) The purity of each component will increase as the solution is distilled multiple times. Fractional distillation is used because it can complete several little distillations, or theoretical plates, in one distillation. The purity of each fraction isolated during the distillation is measured using gas-liquid chromatography, GC. Since each component has a different boiling point and affinity for the liquid stationary phase , each component will have a different response time through the column. This will result in each component having its own peak. -

Distilling Equipment
DISTILLING EQUIPMENT Universal Still MinneMod MinneMod Figgins Reciprocator Pilot Still Low Profile 500 200 Electric EQUIPMENT SPECIFICATIONS Still Pot with CIP Spray Ball Full Copper, 500L Pot Full Copper, 200L Pot Identical twin pots Stainless Steel Still Pot 3’ - 4' shorter than our Steam jacket or electric Bain Steam jacket or electric Single pass vodka purity with fewer Alembic Condenser / comparable 16-plate vodka Marie heat Bain Marie heat bubble plates Spout Electric Heating column system Optional mixing motor Optional mixing motor Increased copper contact Element Various Helmet Options Copper or stainless walled, Optional glass, copper or Faster heat up time Basic System Control Available 8” bubble plate columns can stainless walled, 6” Shorter footprint and height required Panel Full Copper Alembic Arm be assembled on top of pot, bubble plate columns with fewer columns and bubble plates Modular Design allows Gin Basket or as a side column Stainless dephlegmator Either simple pot or hybrid column for scalable bubble plate Dual 10x Bubble Plate sections within the Stainless dephlegmator Columns with 10x CIP Spray Interchangeable helmet operation possible column Balls, Dephlegmator and Spirit Interchangeable helmet and and column options, Hard piped CIP manifold Bubble Plate Sections Locker Bases column options, including including botanical Storage tanks in column base can be Stainless, Stainless Condenser, Parrot botanical infusion chamber infusion chamber Copper or Clear Glass Spout, Base All -

Chem 2219: Exp. #2 Fractional Distillation
Chem 2219: Exp. #2 Fractional Distillation Objective: In this experiment you will learn to separate the components of a solvent mixture (two liquids) by distillation. The boiling point (BP) range and refractive index (RI) will be determined for the three fractions. Gas chromatography will be used to determine the percent composition and purity of the first fraction. * * Gas Chromatography is extremely useful in determining the percent composition/purity of known liquids. Recall the boiling point and refractive index (R.I.) are two useful physical properties of a liquid. They are used for identification purposes. The R.I. is additionally used as a measure of purity of the sample being examined. Reading Assignment: MTOL, pp. 79-83 (distillation theory), 57-62 (gas chromatography) and: OCLT, pp. 254-262 (separation theory), 279-281 (fractional distillation), 139-153 (gas chromatography). Also on CANVAS read and answer prelab questions for – Fractional Distillation and (prelab for next week) Gas Chromatography Concepts: Boiling Point, Condensate, Condensation, Distillate, Distillation, Evaporation, Reflux, Refractive Index, Theoretical Plates, Vaporization Chemicals: Cyclohexane, Toluene, p-Xylene Safety Precautions: Wear chemical splash-proof goggles and appropriate attire at all times. Cyclohexane, toluene and p-xylene are flammable liquids. Hot glassware looks just like cold glassware. Be careful when working with hot glassware! Do not to touch it! Materials: aluminum block, aluminum foil, beaker (100ml), Claisen head adapter, conical vial (5 ml), disposable pipets & bulbs, disposable vials and lids, finger clamps (2), glass wool batting, Hickman still head, hot plate with magnetic stirrer, labels (3-small), magnetic spin vane (large), ringstand, Teflon septum with hole in the center, and thermometer Instruments: Refractometer, Gas Chromatograph (maybe), Magritek desktop NMR (cbolon updated 201030) 1 Chem 2219: Exp.