Distillation of Essential Oils1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Natural Beauty Alchemy : Make Your Own Organic
AN IDEA REMAINS AN IDEA, UNTIL IT IS CRAFTED WITH PASSION! Through the challenges, with a lot of time, effort, and determination, this project came together. For my family, who supported and motivated me when I needed it most; for my beautiful mother, who did everything only a mother can do; for my friends, who were amazing first enthusiasts; and for every natural beauty believer, a heartfelt thank you. CONTENTS INTRODUCTION UNDERSTANDING NATURAL SKIN CARE AND INGREDIENTS Skin Skin Care Categories Ingredients RECIPES Making Your Own Skin Care Products Facial Masks and Scrubs Cleansers and Makeup Removers Toners Evening Oils and Serums Facial Creams and Sunscreen Eye Care Neck Care Body Care Hand Care Foot and Heel Care Hair Care Perfumes and Scented Sprays BUYING COMMERCIAL SKIN CARE PRODUCTS Debated Ingredients Animal-Derived Ingredients Certifications and Seals SUBSTITUTION CHART GLOSSARY DISCLAIMER ACKNOWLEDGMENTS INDEX INTRODUCTION What Is Beauty? Beauty is a perceptual concept that grows with individuals of every society and culture. As we begin to understand what it means and grasp its dimensions, we learn to appreciate it and search for it in every facet of our lives. We also attempt to bring beauty into our daily lives by incorporating beautiful things into our surrounding environment and by trying to beautify our own appearance. Most women, men, and children have an innate desire to look and feel beautiful inside and out, and there is a certain harmony that often links inner and outer beauty and reflects the result to the outside. Each person defines and implements beauty in a different way, but despite the subjectivity, a huge platform remains common ground for most people in search of a more “beautiful” look. -
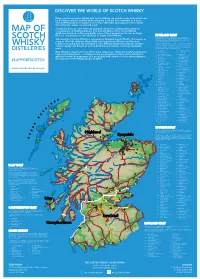
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Hydro-Distillation and Steam Distillation from Aromatic Plants
Hydro-distillation and steam distillation from aromatic plants Sudeep Tandon Scientist Chemical Engineering Division CIMAP, Lucknow HISTORY Written records of herbal distillation are found as early as the first century A.D., and around 1000 A.D., the noted Arab physician and naturalist Ibn Sina also known as Avicenna described the distillation of rose oil from rose petals The ancient Arabian people began to study the chemical properties of essential oils & developed and refined the distillation process Europeans began producing essential oils in the 12th century 1 DISTILLATION ? A process in which a liquid or vapour mixture of two or more substances is separated into its component fractions of desired purity, by the application and removal of heat. In simple terms distillation of aromatic herbs implies vaporizing or liberating the oils from the trichomes / plant cell membranes of the herb in presence of high temperature and moisture and then cooling the vapour mixture to separate out the oil from water. It is the most popular widely used and cost effective method in use today for producing majority of the essential oils throughout the world Distillation is an art and not just a “Chemical" process that is reliant upon many factors for successful quality oil production. BASIC SCIENTIFIC PRINCIPLES INVOLVED IN THE PROCESS To convert any liquid into a vapour we have to apply energy in form of heat called as latent heat of vaporization A liquid always boils at the temperature at which its vapour pressure equals the atmospheric / surrounding pressure For two immiscible liquids the total vapour pressure of the mixture is always equal to the sum of their partial pressures The composition of the mixture will be determined by the concentration of the individual components into its partial pressure As known the boiling point of most essential oil components exceeds that of water and generally lies between 150 – 300oC 2 If a sample of an essential oil having a component ‘A’ having boiling point for example 190oC and the boiling point of the water is 100oC. -

Perfume Engineering Perfume Engineering Design, Performance & Classification
Perfume Engineering Perfume Engineering Design, Performance & Classification Miguel A. Teixeira, Oscar Rodríguez, Paula Gomes, Vera Mata, Alírio E. Rodrigues Laboratory of Separation and Reaction Engineering (LSRE) Associate Laboratory Department of Chemical Engineering Faculty of Engineering of University of Porto Porto, Portugal P. Gomes and V. Mata are currently at i-sensis company S. João da Madeira, Portugal AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Butterworth-Heinemann is an imprint of Elsevier Butterworth-Heinemann is an imprint of Elsevier The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK 225 Wyman Street, Waltham, MA 02451, USA First published 2013 Copyright r 2013 Elsevier Ltd. All rights reserved No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

A Guide to Stills & Distilling
A GUIDE TO STILLS & DISTILLING LEADERS IN HOME DISTILLING AND FILTRATION SYSTEMS THAT MEET AND EXCEED EVEN COMMERCIAL STANDARDS. www.stillspirits.com Reorder: 72648 V1 Reorder: With over 25 years in the Air Still A compact, sleek stainless steel still for those who want a no fuss way market, Still Spirits is a leader of producing 1 L (1 US qt) of 40% ABV spirit at a time. Distils alcohol at 60% strength. Used with a small 10 L (2.6 US Gal) fermenter making this in home distilling and filtration system easy to move and operate from any kitchen, boat or campervan. systems that meet and exceed AVERAGE USE even commercial standards. DIFFICULTY Basic CAPACITY 4 L (1 US Gal) The range features: TIME 7- 10 day Fermentation Premium spirits with equivalent commercial quality Approx 2 hours distilling per 1 L (1 US qt) of 40% alcohol at a fraction of the price. YIELD 2 L (2 US qt) of 40% ABV alcohol per 8 L Homemade spirits in as little as 7 days. (2 US Gal) wash Distilled alcohol from a wash made simply using sugar, PURITY Distils at 60% ABV (120 US proof) before being watered down to 40% (80 US proof) yeast, carbon and drinkable water. Homemade spirit flavoured with your choice of Still Spirits spirit or liqueur essences. QUICK GUIDE TO DISTILLING FEATURES WITH THE AIR STILL 1. Wash – Make an 8 L (2 US Gal) wash by • Easy to operate, set up and use. mixing water, sugar, carbon and yeast With no hoses or complicated in the fermenter. -

Canadian Whisky, American Whiskey & Bourbon – Corn, Rye, Malt
TITLE ADDING BEVERAGE ALCOHOL DISTILLATION TO AN EXISTING BREWERY Presented by Stephen Harkness, P.Eng., President, cemcorp LTD. MBAC District Ontario Spring Technical Meeting, April 23, 2015 HISTORY CANADIAN DISTILLERS 2015 RETAIL STORE POT STILL DIFFERENCES DIFFERENCES BREWERY VS. DISTILLERY 1. NOMENCLATURE/ 5. DISTILLATION TERMINOLOGY PROCESS 2. RAW MATERIAL 6. FINAL PRODUCT 3. MASHING/COOKING 7. BUILDING DESIGN 4. FERMENTATION 8. LICENSING PROCESS REQUIREMENTS NOMENCLATURE – PROOF STRENGTH 1. NOMENCLATURE PROOF STRENGTH U.S. DEFINITION: 1 PROOF = 0.5% A/V i.e. 200 PROOF = 100% A/V BRITISH DEFINITION: 100 PROOF = 57.06% A/V i.e. 175.25 PROOF = 100% A/V METRIC DEFINITION: 1°GL (GAY LUSSAC) = 1% A/V i.e. 100°GL = 100% a/v NOMENCLATURE 1. NOMENCLATURE • BREWERS MEASURE VOLUMES IN HECTOLITRES 1 HL = 0.85 US Barrels = 0.61 UK Barrels (BBL) DISTILLERS MEASURE VOLUMES IN LAA (LITRES ABSOLUTE ALCOHOL) OR PROOF GALLONS • CANADIAN CASE OF BEER IS 24 BOTTLES 341 ml CASE OF LIQUOR IS 12 BOTTLES 750 ml • CANADIAN BREWERS STATE STRENGTH AS %A/V (AMERICAN BREWERS STATE STRENGTH AS %A/W) DISTILLERS STATE STRENGTH AS % OF LAA • REFLUX, DEPHLEGMATOR, DOWNPIPE, WEIR, CONGENERS RAW MATERIAL 2. RAW MATERIAL WHISKY/WHISKEY, including CANADIAN WHISKY, AMERICAN WHISKEY & BOURBON – CORN, RYE, MALT SCOTCH WHISKY AND IRISH WHISKEY – MALT RUM – MOLASSES OR CANE JUICE VODKA – ANY STARCH OR SUGAR GRAPPA, LIQUEURS, BRANDIES SNAPPS - FRUIT MASHING/COOKING 3. MASHING/COOKING 1. BATCH COOKERS 2. CONTINUOUS COLUMNAR COOKERS 3. U-TUBE CONTINUOUS JET COOKERS 4. EXTRUDERS FERMENTATION 4. FERMENTATION BREWERIES FERMENT 5 – 20 DAYS @ 12°C. – 20° C. -

Azeotropes As Powerful Tool for Waste Minimization in Industry and Chemical Processes
molecules Review Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes Federica Valentini and Luigi Vaccaro * Laboratory of Green S.O.C., Dipartimento di Chimica, Biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto 8, 06123 Perugia, Italy; [email protected] * Correspondence: [email protected]; Tel./Fax: +39-07-5585-5541 Academic Editor: Derek J. McPhee Received: 17 October 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: Aiming for more sustainable chemical production requires an urgent shift towards synthetic approaches designed for waste minimization. In this context the use of azeotropes can be an effective tool for “recycling” and minimizing the large volumes of solvents, especially in aqueous mixtures, used. This review discusses the implementation of different kinds of azeotropic mixtures in relation to the environmental and economic benefits linked to their recovery and re-use. Examples of the use of azeotropes playing a role in the process performance and in the purification steps maximizing yields while minimizing waste. Where possible, the advantages reported have been highlighted by using E-factor calculations. Lastly azeotrope potentiality in waste valorization to afford value-added materials is given. Keywords: azeotrope; waste minimization; solvent recovery; azeotropic distillation; process design; waste valorization 1. Introduction The materials not incorporated into the final desired product and the energy required for conducting a process can be considered most trivially as the major direct origin of “waste” associated to chemical production. In addition, the possible formation of undesired isomers or byproducts also results in the generation of costly waste, that being in some cases hazardous, can limit the final application of the main product. -

Sustainability Report 2019-2020
Sustainability Report 2019-2020 The Origin of the These notebooks were used by Tadaka Kainosho, the founder of Takasago International Corporation, when he was studying perfumery Corporate Philosophy in Europe. He put down everything he learned in France and Switzerland in these notebooks. Containing pages of writing in French and German, they give a sense of the young Tadaka’s passion as well as his keen interest in the technology. The notebooks hold a great deal of meaning for Takasago International Corporation, which Tadaka later founded, as they provide the foundation of the company’s corporate philosophy of technology-based development. In 1920, Tadaka founded a company specializing in the manufacture of aroma ingredients, one of the first of its kind in Japan, based on a strong belief that fragrances and flavors enrich people’s lives and can thus contribute to society. The company has grown into a global enterprise and yet still maintains the passion of its founder to contribute to society through technology, which remains unchanged even as the world continues to evolve. In order to fulfill our mission to deliver to countless people products that can increase their enjoyment of life, we will drive our global business forward, aiming to become a world-leading flavors and fragrances supplier. “Kainosho notebooks,” where Tadaka wrote about the research in Europe (center/right) and a photo taken during the training at a natural aroma manufacturer in Grasse, South France (left) The Tokyo factory (currently the head office building in Kamata, Ota-ku) at the time of the Takasago International Corporation foundation and the founder Tadaka Kainosho marks its 100th birthday in 2020. -

Perfume Composition Containing (4R)-Cis-4-Methyl-2-Substituted-Tetrahydro-2H-Pyran Derivative and Method for Improving Fragrance by Using It
~™ II 1 1 III II IMI II II I Ml II I II I II (19) J European Patent Office Office europeen des brevets (11) EP 0 770 670 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication:ation: (51) |nt. CI.6: C1 1 B 9/00, A61 K 7/46 02.05.1997 Bulletin 1997/18 (21) Application number: 96116354.0 (22) Date of filing: 11.10.1996 (84) Designated Contracting States: • Yamamoto, Takeshi, CH DE FR LI NL c/o Takasago Int. Corporation Hiratsuka-shi, Kanagawa (JP) (30) Priority: 13.10.1995 JP 291932/95 (74) Representative: Grunecker, Kinkeldey, (71 ) Applicant: Takasago International Corporation Stockmair & Schwanhausser Tokyo 108 (JP) Anwaltssozietat Maximilianstrasse 58 (72) Inventors: 80538 Munchen (DE) • Matsuda, Hiroyuki, c/o Takasago Int. Corporation Hiratsuka-shi, Kanagawa (JP) (54) Perfume composition containing (4R)-cis-4-methyl-2-substituted-tetrahydro-2H-pyran derivative and method for improving fragrance by using it (57) A perfume composition which contains a (4R)-cis-4-methyl-2-substituted-tetrahydro-2H-pyran derivative repre- sented by formula (I): wherein ^ represents a single or double bond, and a method for improving fragrance by using this perfume composition. CM < O io o r»- o Q_ LU Printed by Rank Xerox (UK) Business Services 2.14.3/3.4 EP 0 770 670 A2 Description FIELD OF THE INVENTION 5 This invention relates to a perfume composition containing a (4R)-cis-4-methyl-2-substituted-tetrahydro-2H-pyran derivative, which has a high chemical purity and a high optical purity and can impart a refreshing, light and rose green feel to the fragrance of the perfume composition, and a method for improving a fragrance.