Chem 2219: Exp. #2 Fractional Distillation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction of Wax from Sorghum Bran •
i EXTRACTION OF WAX FROM SORGHUM BRAN • 1 HSIEN-WEN HSU <& . ***£/ B. S., National Taiwan University, China, 1951 A THESIS » submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE - Department of Chemical Engineering KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE - 1955 » k Lo i~i ii Q^cu^v^Js TABLE OF CONTENTS INTRODUCTION 1 PREVIOUS WORK 3 MATERIALS 6 GLASS SOXHLET EXTRACTION AND ANALYSIS OF WAX AND OIL IN MISCELLA 7 Procedure of Using Soxhlet Extractor 7 Separation of Wax from Oil by the Acetone Method.. 8 Summary of Results 9 PILOT PLANT EXTRACTION 15 SEPARATION OF WAX AND OIL 16 Effect of Temperature in the Acetone Method 16 Urea Complex Method of Separation. 17 Theory 17 Experimental Procedure 22 Summary of Results 26 COST ESTIMATION OF A WAX RECOVERY PLANT 29 DISCUSSION 30 CONCLUSIONS 35 ACKNOWLEDGMENTS 36 BIBLIOGRAPHY 37 APPENDIX 39 INTRODUCTION i One of the functions of the Agricultural Experiment Station is the never ending search for means of better and fuller utilization of the agri- cultural products that are of particular interest in the state of Kansas. In line with this broad objective, the extraction of economically valuable lipid materials from the sorghum bran has been studied in several theses in the Departments of Chemical Engineering and Chemistry in recent years. Generally the emphasis has b. en placed upon either the chemical and physical properties of these lipides or the equipment design and performance for the solvent ex- traction operation. A brief review of the previous work is presented in the ensuing section. -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Distillation 65 Chem 355 Jasperse DISTILLATION
Distillation 65 Chem 355 Jasperse DISTILLATION Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize. The vapors produced are subsequently passed through a water-cooled condenser. Upon cooling, the vapor returns to it’s liquid phase. The liquid can then be collected. The ability to separate mixtures of liquids depends on differences in volatility (the ability to vaporize). For separation to occur, the vapor that is condensed and collected must be more pure than the original liquid mix. Distillation can be used to remove a volatile solvent from a nonvolatile product; to separate a volatile product from nonvolatile impurities; or to separate two or more volatile products that have sufficiently different boiling points. Vaporization and Boiling When a liquid is placed in a closed container, some of the molecules evaporate into any unoccupied space in the container. Evaporation, which occurs at temperatures below the boiling point of a compound, involves the transition from liquid to vapor of only those molecules at the liquid surface. Evaporation continues until an equilibrium is reached between molecules entering and leaving the liquid and vapor states. The pressure exerted by these gaseous molecules on the walls of the container is the equilibrium vapor pressure. The magnitude of this vapor pressure depends on the physical characteristics of the compound and increases as temperature increases. In an open container, equilibrium is never established, the vapor can simply leave, and the liquid eventually disappears. But whether in an open or closed situation, evaporation occurs only from the surface of the liquid. -
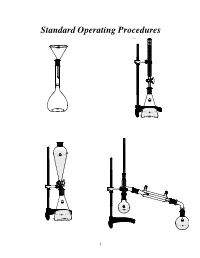
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

Experiment 2 — Distillation and Gas Chromatography
Chem 21 Fall 2009 Experiment 2 — Distillation and Gas Chromatography _____________________________________________________________________________ Pre-lab preparation (1) Read the supplemental material on distillation theory and techniques from Zubrick, The Organic Chem Lab Survival Manual, and the section on Gas Chromatography from Fessenden, Fessenden, and Feist, Organic Laboratory Techniques, then read this handout carefully. (2) In your notebook, write a short paragraph summarizing what you will be doing in this experiment and what you hope to learn about the efficiencies of the distillation techniques. (3) Sketch the apparatus for the simple and fractional distillations. Your set-up will look much like that shown on p 198 of Zubrick, except that yours will have a simple drip tip in place of the more standard vacuum adaptor. (4) Look up the structures and relevant physical data for the two compounds you will be using. What data are relevant? Read the procedure, think about the data analysis, and decide what you need. (5) Since you have the necessary data, calculate the log of the volatility factor (log α) that you will need for the theoretical plate calculation. Distillation has been used since antiquity to separate the components of mixtures. In one form or another, distillation is used in the manufacture of perfumes, flavorings, liquors, and a variety of other organic chemicals. One of its most important modern applications is in refining crude oil to make fuels, lubricants, and other petrochemicals. The first step in the refining process is separation of crude petroleum into various hydrocarbon fractions by distillation through huge fractionating columns, called distillation towers, that are hundreds of feet high. -

SAFETY DATA SHEET Cyclohexane BDH1111
SAFETY DATA SHEET Cyclohexane BDH1111 Version 1.3 Revision Date 03/25/2015 Print Date 05/08/2015 SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Cyclohexane MSDS Number : 000000011713 Product Use Description : Solvent Manufactured for : VWR International LLC Radnor Corporate Center Building One Suite 200 100 Matsonford Road Radnor PA 19087 For more information call : (Monday-Friday,8.00am-5:00pm) 1-800-932-5000 In case of emergency call : (24 hours/day, 7 days/week) 1-800-424-9300(USA Only) For Transportation Emergencies: 1-800-424-9300 (CHEMTREC - Domestic) 1-613-996-6666 (CANUTEC - Canada) SECTION 2. HAZARDS IDENTIFICATION Emergency Overview Form : liquid, clear Color : colourless Odor : mild sweet Page 1 / 15 SAFETY DATA SHEET Cyclohexane BDH1111 Version 1.3 Revision Date 03/25/2015 Print Date 05/08/2015 Classification of the substance or mixture Classification of the substance : Flammable liquids, Category 2 or mixture Skin irritation, Category 2 Specific target organ toxicity - single exposure, Category 3, Central nervous system Aspiration hazard, Category 1 GHS Label elements, including precautionary statements Symbol(s) : Signal word : Danger Hazard statements : Highly flammable liquid and vapour. May be fatal if swallowed and enters airways. Causes skin irritation. May cause drowsiness and dizziness. Precautionary statements : Prevention : Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Keep container tightly closed. Ground/bond container and receiving equipment. Use explosion-proof electrical/ ventilating/ lighting/ equipment. Use only non-sparking tools. Take precautionary measures against static discharge. Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray. Wash skin thoroughly after handling. Use only outdoors or in a well-ventilated area. -

Safety Data Sheet: Cyclohexane
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Cyclohexane ROTISOLV® ≥99,9 %, UV/IR-Grade article number: CP81 date of compilation: 2018-05-03 Version: 2.0 en Revision: 2020-10-06 Replaces version of: 2018-05-03 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Cyclohexane ROTISOLV® ≥99,9 %, UV/IR-Grade Article number CP81 Registration number (REACH) 01-2119463273-41-xxxx Index No 601-017-00-1 EC number 203-806-2 CAS number 110-82-7 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: laboratory chemical laboratory and analytical use 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone: +49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data : Department Health, Safety and Environment sheet: e-mail (competent person): [email protected] 1.4 Emergency telephone number Name Street Postal code/ Telephone Website city National Poisons Inform- Beaumont Road Dublin 9 01 809 2166 https://www.poisons.ie/ ation Centre Beaumont Hospital SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Classification acc. to GHS Section Hazard class Hazard class and cat- Hazard egory state- ment 2.6 flammable liquid (Flam. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -
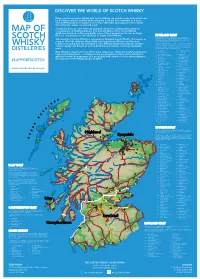
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Laboratory Rectifying Stills of Glass 2
» LABORATORY RECTIFYING STILLS OF GLASS 2 By Johannes H. Bruun 3 and Sylvester T. Schicktanz 3 ABSTRACT A complete description is given of a set of all-glass rectifying stills, suitable for distillation at pressures ranging from atmospheric down to about 50 mm. The stills are provided with efficient bubbling-cap columns containing 30 to 60 plates. Adiabatic conditions around the column are maintained by surrounding it with a jacket provided with a series of independent electrical heating units. Suitable means are provided for adjusting or maintaining the reflux ratio at the top of the column. For the purpose of conveniently obtaining an accurate value of the true boiling points of the distillates a continuous boiling-point apparatus is incor- porated in the receiving system. An efficient still of the packed-column type for distillations under pressures less than 50 mm is also described. Methods of operation and efficiency tests are given for the stills. CONTENTS Page I. Introduction 852 II. General features of the still assembly 852 III. Still pot 853 IV. Filling tube and heater for the still pot 853 V. Rectifying column 856 1. General features 856 2. Large-size column 856 3. Medium-size column 858 4. Small-size column 858 VI. Column jacket 861 VII. Reflux regulator 861 1. With variable reflux ratio 861 2. With constant reflux ratio 863 VIII. The continuous boiling-point apparatus 863 IX. Condensers and receiver 867 X. The mounting of the still 867 XI. Manufacture and transportation of the bubbling-cap still 870 XII. Operation of the bubbling-cap still 870 XIII. -

Cyclohexane Oxidation Continues to Be a Challenge Ulf Schuchardt A,∗, Dilson Cardoso B, Ricardo Sercheli C, Ricardo Pereira A, Rosenira S
Applied Catalysis A: General 211 (2001) 1–17 Review Cyclohexane oxidation continues to be a challenge Ulf Schuchardt a,∗, Dilson Cardoso b, Ricardo Sercheli c, Ricardo Pereira a, Rosenira S. da Cruz d, Mário C. Guerreiro e, Dalmo Mandelli f , Estevam V. Spinacé g, Emerson L. Pires a a Instituto de Qu´ımica, Universidade Estadual de Campinas, P.O. Box 6154, 13083-970 Campinas, SP, Brazil b Depto de Eng. Qu´ımica, Universidade Federal de São Carlos, 13565-905 São Carlos, SP, Brazil c College of Chemistry, University of California, Berkeley, CA 94720, USA d Depto Ciências Exatas e Tecnológicas, Universidade Estadual de Santa Cruz, 45650-000 Ilhéus, BA, Brazil e Universidade Federal de Lavras, Lavras, MG, Brazil f Instituto de Ciências Biológicas e Qu´ımicas, PUC-Campinas, 13020-904 Campinas, SP, Brazil g Sup. Caracterização Qu´ımica, IPEN, 05508-900 São Paulo, SP, Brazil Received 3 October 2000; received in revised form 21 December 2000; accepted 28 December 2000 Abstract Many efforts have been made to develop new catalysts to oxidize cyclohexane under mild conditions. Herein, we review the most interesting systems for this process with different oxidants such as hydrogen peroxide, tert-butyl hydroperoxide and molecular oxygen. Using H2O2, Na-GeX has been shown to be a most stable and active catalyst. Mesoporous TS-1 and Ti-MCM-41 are also stable, but the use of other metals such as Cr, V, Fe and Mo leads to leaching of the metal. Homogeneous systems based on binuclear manganese(IV) complexes have also been shown to be interesting. When t-BuOOH is used, the active systems are those phthalocyanines based on Ru, Co and Cu and polyoxometalates of dinuclear ruthenium and palladium.