A Demonstration of the Sublimation Process and Its Effect on Students’ Conceptual Understanding of the Sublimation Concept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dry Ice Blasting
Dry Ice Blasting The Dry Ice Blasting method can reduce the overall time spent on sanding, scraping or scrub- bing with solvents, acids and other cleaning agents. With no secondary waste generation there is no need for disposal of hazardous waste. This saves time and money while increasing worker safety. Online Cleaning of Fin Dry Ice Blasting can safely and effectively clean contaminants that plug and foul fin fan heat Fan Heat Exchangers exchangers. Dry Ice Blasting can be performed while the system is online so no costly downtime is required. Cleaning will increase airflow which will lower approach temperatures and increase efficiency. The dry nature of the process will mitigate the potential for harming motors, bearings or instrumentation. Other Applications May The demand to keep the equipment running often leads to deferred cleaning and maintenance, Also Benefit reduced efficiency and in some cases, outages caused by flashover. Dry ice blast cleaning can provide a non-conductive cleaning process that may allow equipment to be cleaned in-place without cool down or disassembly. This includes generators, turbines, stators, rotors, compressors and boilers. Industrial cleaning applications exist in markets as diverse as food & beverage equipment clean-up, pharmaceutical production, aerospace surfaces & components, and foundry core- making machinery. A Leading Supplier of Working with Linde Services offers you product reliability from an industry leader in carbon Carbon Dioxide dioxide applications. This includes: → A focus on safety -

Physical Changes, Chemical Changes, and How to Tell the Difference
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Physical Changes, Chemical Changes, and How to Tell the Difference Presented by: Adam Boyd December 5, 2012 6:30 p.m. – 8:00 p.m. Eastern time 1 Introducing today’s presenter… Adam Boyd Senior Education Associate Office of K–8 Science American Chemical Society 2 American Chemical Society Physical Changes, Chemical Changes, and How to Tell the Difference Adam M. Boyd Education Division American Chemical Society Our Goals Inquiry Based Activities – Clues of chemical change How to Science Background distinguish? – Chemical and Physical Properties – Chemical and Physical Change American Chemical Society 4 IYC Kits www.acs.org/iyckit American Chemical Society 5 IYC Kit Lesson Components 1. Lesson Summary 1 2. Key Concepts 3. Safety 2 4. The chemistry continues 5. Scientist introduction 6. Teacher demonstration(s) 3 7. Student activity Student activity sheet 8. Class discussion 9. Teacher demonstration 10. Application 4 American Chemical Society 6 IYC Kit Classic clues of chemical change? American Chemical Society 7 IYC Kit 1. Production of a gas Classic clues of chemical change? American Chemical Society 8 IYC Kit 1. Production of a gas Classic clues of chemical change? 2. Color change American Chemical Society 9 IYC Kit 1. Production of a gas Classic clues of chemical change? 2. Color change 3. Formation of a precipitate American Chemical Society 10 IYC Kit 1. Production of a gas Classic clues of chemical change? 2. Color change 3. Formation of a precipitate 4. Temperature change American Chemical Society 11 Chemical change or Physical change? Chemical change or Physical change? Chemical Change Physical Change IYC Kit 1. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -
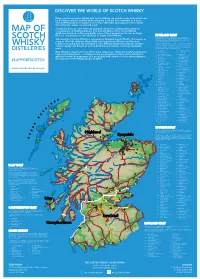
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Hydro-Distillation and Steam Distillation from Aromatic Plants
Hydro-distillation and steam distillation from aromatic plants Sudeep Tandon Scientist Chemical Engineering Division CIMAP, Lucknow HISTORY Written records of herbal distillation are found as early as the first century A.D., and around 1000 A.D., the noted Arab physician and naturalist Ibn Sina also known as Avicenna described the distillation of rose oil from rose petals The ancient Arabian people began to study the chemical properties of essential oils & developed and refined the distillation process Europeans began producing essential oils in the 12th century 1 DISTILLATION ? A process in which a liquid or vapour mixture of two or more substances is separated into its component fractions of desired purity, by the application and removal of heat. In simple terms distillation of aromatic herbs implies vaporizing or liberating the oils from the trichomes / plant cell membranes of the herb in presence of high temperature and moisture and then cooling the vapour mixture to separate out the oil from water. It is the most popular widely used and cost effective method in use today for producing majority of the essential oils throughout the world Distillation is an art and not just a “Chemical" process that is reliant upon many factors for successful quality oil production. BASIC SCIENTIFIC PRINCIPLES INVOLVED IN THE PROCESS To convert any liquid into a vapour we have to apply energy in form of heat called as latent heat of vaporization A liquid always boils at the temperature at which its vapour pressure equals the atmospheric / surrounding pressure For two immiscible liquids the total vapour pressure of the mixture is always equal to the sum of their partial pressures The composition of the mixture will be determined by the concentration of the individual components into its partial pressure As known the boiling point of most essential oil components exceeds that of water and generally lies between 150 – 300oC 2 If a sample of an essential oil having a component ‘A’ having boiling point for example 190oC and the boiling point of the water is 100oC. -

NASA Spacecraft Observes Further Evidence of Dry Ice Gullies on Mars 11 July 2014, by Guy Webster
NASA spacecraft observes further evidence of dry ice gullies on Mars 11 July 2014, by Guy Webster "As recently as five years ago, I thought the gullies on Mars indicated activity of liquid water," said lead author Colin Dundas of the U.S. Geological Survey's Astrogeology Science Center in Flagstaff, Arizona. "We were able to get many more observations, and as we started to see more activity and pin down the timing of gully formation and change, we saw that the activity occurs in winter." Dundas and collaborators used the High Resolution Imaging Science Experiment (HiRISE) camera on MRO to examine gullies at 356 sites on Mars, beginning in 2006. Thirty-eight of the sites showed active gully formation, such as new channel segments and increased deposits at the downhill end of some gullies. Using dated before-and-after images, researchers determined the timing of this activity coincided with This pair of images covers one of the hundreds of sites seasonal carbon-dioxide frost and temperatures on Mars where researchers have repeatedly used the that would not have allowed for liquid water. High Resolution Imaging Science Experiment (HiRISE) camera on NASA's Mars Reconnaissance Orbiter to study changes in gullies on slopes. Credit: NASA/JPL- Frozen carbon dioxide, commonly called dry ice, Caltech/Univ. of Arizona does not exist naturally on Earth, but is plentiful on Mars. It has been linked to active processes on Mars such as carbon dioxide gas geysers and lines on sand dunes plowed by blocks of dry ice. One Repeated high-resolution observations made by mechanism by which carbon-dioxide frost might NASA's Mars Reconnaissance Orbiter (MRO) drive gully flows is by gas that is sublimating from indicate the gullies on Mars' surface are primarily the frost providing lubrication for dry material to formed by the seasonal freezing of carbon dioxide, flow. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Distillation of Essential Oils1
WEC310 Distillation of Essential Oils1 Elise V. Pearlstine 2 A short history of essential oils many industries and in new applications as awareness of the benefit of naturally derived products grows. Essential oils are volatile, aromatic oils obtained from plants and used for fragrance, flavoring, and health and beauty applications. Historically, aromatic plants provided important ingredients for perfumes, incense, and cosmetics. They have also been used for ritual purposes and in cooking and medicine. Egyptians used aromatic plant materials to preserve mummies, the Ayurvedic literature of India includes many references to scented substances, ancient Chinese herbalists valued them for their curative properties, and royalty used rare aromatics to perfume themselves and their surroundings. Distillation became an important An eighteenth century still from an old method of obtaining the healing and fragrant Figure 1. monograph by Gildemeister. components of various plants and was well-studied beginning in the 18th and continuing in the 19th Plant anatomy and structure as they centuries (Figure 1). In the 1900s, during the time of relate to essential oil production the industrial revolution, component parts of many essential oils were identified. These components An essential oil is the volatile material derived could then be synthesized for use in perfume and from plant material by a physical process. The plant flavor industries. The art of using essential oils material is usually aromatic and of a single botanical declined during this time but experienced a re-birth in species and form; some essential oil plants have a Europe with aromatherapy later in the century. In different chemical makeup depending on the variety recent years, the use of essential oils has increased in of plant, and the essential oils are correspondingly unique. -

It's Just a Phase!
Bay Area Scientists in School Presentation Plan Lesson Name It’s just a phase!______________ Presenter(s) Kevin Metcalf, David Ojala, Melanie Drake, Carly Anderson, Hilda Buss, Lin Louie, Chris Jakobson California Standards Connection(s): 3rd Grade – Physical Science 3-PS-Matter has three states which can change when energy is added or removed. Next Generation Science Standards: 2nd Grade – Physical Science 2-PS1-1. Plan and conduct an investigation to describe and classify different kinds of materials by their observable properties. 2-PS1-4. Construct an argument with evidence that some changes caused by heating or cooling can be reversed and some cannot. Science & Engineering Practices Disciplinary Core Ideas Crosscutting Concepts Planning and carrying out PS1.A: Structure and Properties Patterns investigations to answer of Matter ・Patterns in the natural and questions or test solutions to ・Different kinds of matter exist human designed world can be problems in K–2 builds on prior and many of them can be observed. (2-PS1-1) experiences and progresses to either solid or liquid, simple investigations, based on depending on temperature. Cause and Effect fair tests, which provide data to Matter can be described and ・Events have causes that support explanations or design classified by its observable generate observable patterns. solutions. properties. (2-PS1-1) (2-PS1-4) ・Plan and conduct an ・Different properties are suited ・Simple tests can be designed to investigation collaboratively to to different purposes. (2-PS1- gather evidence to support or produce data to serve as the 2), (2-PS1-3) refute student ideas about basis for evidence to answer a ・A great variety of objects can causes. -

ACIDS and BASES Unique Properties of Dry Ice
ACIDS AND BASES Unique Properties of Dry Ice OVERVIEW: Students observe properties of dry ice. For example, whether dry ice can change the color of a basic solution that contains an indicator OBJECTIVE: To review basic principles of matter and how this relates to every day objects. Develop concepts and nature of acids and bases and relate this to common household items. GRADE LEVEL: 6-7 OHIO STANDARDS: PS7 Grade 7 Physical Science: The properties of matter are determined by the arrangement of atoms. TIME: 30-45 minutes VOCABULARY: acid, base, organic, inorganic, indicator, pH, hydrogen ion, molecule, CO2, dry ice MATERIALS: (per group of 5-6 students) *Block of dry ice *8 Beakers—600 ml tall with wide mouth *1 Graduated cylinder-10ml *1 stirring rod IMPORTANT: *Gloves.—cotton and latex Read all instructions before *Safety glasses proceeding *Indicator Solutions *pH paper *Balloons *1 pint Ammonia * 1 pint Vinegar DEVELOPED BY: Bob Maloney, Analytical Chemist, BP Chemicals INDICATOR SOLUTIONS: 100-250 ml of one or more of the following: *Grape juice concentrate *Cherry Juice *Beet Juice *Red Cabbage Juice VARIATION: 10-15 ml of one of the following: Thymolphthalein solution: (To prepare 100 ml of stock solution, dissolve 0.04g of thymolphthalein in 50 ml of 95% ethyl alcohol (ethanol) and dilute the resulting solution to 100ml with water. Alternatively, “Disappearing Ink” which is sold in toy stores can be used). Phenolphthalein solution (To prepare 10ml stock solution, dissolve 0.05g phenolphthalein in 50 ml of 95% ethyl alcohol and dilute the resulting solution to 100ml with water. Alternatively, crush two or three EX-Lax tablets and cover with rubbing alcohol and mix. -

42 the Reaction Between Zinc and Copper Oxide
The reaction between zinc and copper oxide 42 In this experiment copper(II) oxide and zinc metal are reacted together. The reaction is exothermic and the products can be clearly identified. The experiment illustrates the difference in reactivity between zinc and copper and hence the idea of competition reactions. Lesson organisation This is best done as a demonstration. The reaction itself takes only three or four minutes but the class will almost certainly want to see it a second time. The necessary preparation can usefully be accompanied by a question and answer session. The zinc and copper oxide can be weighed out beforehand but should be mixed in front of the class. If a video camera is available, linked to a TV screen, the ‘action’ can be made more dramatic. Apparatus and chemicals Eye protection Bunsen burner • Heat resistant mat Tin lid Beaker (100 cm3) Circuit tester (battery, bulb and leads) (Optional) Safety screens (Optional) Test-tubes, 2 (Optional – see Procedure g) Test-tube rack Access to a balance weighing to the nearest 0.1 g The quantities given are for one demonstration. Copper(II) oxide powder (Harmful, Dangerous for the environment), 4 g Zinc powder (Highly flammable, Dangerous for the environment), 1.6 g Dilute hydrochloric acid, approx. 2 mol dm-3 (Irritant), 20 cm3 Zinc oxide (Dangerous for the environment), a few grams Copper powder (Low hazard), a few grams. Concentrated nitric acid (Corrosive, Oxidising), 5 cm3 (Optional – see Procedure g) Technical notes Copper(II) oxide (Harmful, Dangerous for the environment) Refer to CLEAPSS® Hazcard 26. Zinc powder (Highly flammable, Dangerous for the environment) Refer to CLEAPSS® Hazcard 107 Dilute hydrochloric acid (Irritant at concentration used) Refer to CLEAPSS® Hazcard 47A and Recipe Card 31 Concentrated nitric acid (Corrosive, Oxidising) Refer to CLEAPSS® Hazcard 67 Copper powder (Low hazard) Refer to CLEAPSS® Hazcard 26 Zinc oxide (Dangerous for the environment) Refer to CLEAPSS® Hazcard 108.