Simulation and Analysis of a Middle Vessel Batch Distillation Column
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Computer Simulation of a Multicomponent, Multistage Batch Distillation Process
COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS By BRUCE EARL BAUGHER /J Bachelor of Science in Chemical Engineering Oklahoma State University Stillwater, Oklahoma 1985 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 1988 \\,~s~ ~ \'\~~ B~~~to' <:.o?. ·;)._, ~ r COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS Thesis Approved: Thesis Advisor U.bJ ~~- ~MJYl~ Dean of the GradU~te College ABSTRACT The material and energy balance equations for a batch distillation column were derived and a computer simulation was developed to solve these equations. The solution follows a modified Newton-Raphson approach for inverting and solving the matrices of material and energy balance equations. The model is unique in that it has been designed to handle hypothetical hydrocarbon components. The simulation can handle columns up to 50 trays and systems of up to 100 components. The model has been used to simulate True Boiling Point (TBP) diagrams for a variety of crude oils. This simulation is also applicable to simple laboratory batch distillations. The model was designed to accurately combine data files to simulate actual crude blending procedures. The model will combine files and calculate the results quickly and easily. The simulation involves removing material from the column at steady-state. The removal fraction is made small enough to approach continuity. This simulation can be adapted for use on microcomputers, although it will require extensive computation time. ACKNOWLEDGMENTS It has been a great pleasure to work with my thesis advisor, Dr. -
2019 Scotch Whisky
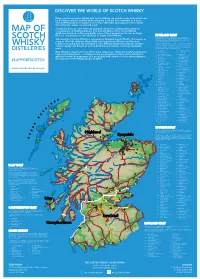
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

United States Patent (10) Patent No.: US 9,586,922 B2 Wood Et Al
USOO9586922B2 (12) United States Patent (10) Patent No.: US 9,586,922 B2 Wood et al. (45) Date of Patent: Mar. 7, 2017 (54) METHODS FOR PURIFYING 9,388,150 B2 * 7/2016 Kim ..................... CO7D 307/48 5-(HALOMETHYL)FURFURAL 9,388,151 B2 7/2016 Browning et al. 2007/O161795 A1 7/2007 Cvak et al. 2009, 0234142 A1 9, 2009 Mascal (71) Applicant: MICROMIDAS, INC., West 2010, 0083565 A1 4, 2010 Gruter Sacramento, CA (US) 2010/0210745 A1 8/2010 McDaniel et al. 2011 O144359 A1 6, 2011 Heide et al. (72) Inventors: Alex B. Wood, Sacramento, CA (US); 2014/01 00378 A1 4/2014 Masuno et al. Shawn M. Browning, Sacramento, CA 2014/O1878O2 A1 7/2014 Mikochik et al. (US);US): Makoto N. Masuno,M. s Elk Grove, s 2015/02668432015,0203462 A1 9/20157/2015 CahanaBrowning et etal. al. CA (US); Ryan L. Smith, Sacramento, 2016/0002190 A1 1/2016 Browning et al. CA (US); John Bissell, II, Sacramento, 2016,01681 07 A1 6/2016 Masuno et al. CA (US) 2016/02O7897 A1 7, 2016 Wood et al. (73) Assignee: MICROMIDAS, INC., West FOREIGN PATENT DOCUMENTS Sacramento, CA (US) CN 101475544. A T 2009 (*) Notice: Subject to any disclaimer, the term of this N 1939: A 658 patent is extended or adjusted under 35 DE 635,783 C 9, 1936 U.S.C. 154(b) by 0 days. EP 291494 A2 11, 1988 EP 1049657 B1 3, 2003 (21) Appl. No.: 14/852,306 GB 1220851 A 1, 1971 GB 1448489 A 9, 1976 RU 2429234 C2 9, 2011 (22) Filed: Sep. -

Hydro-Distillation and Steam Distillation from Aromatic Plants
Hydro-distillation and steam distillation from aromatic plants Sudeep Tandon Scientist Chemical Engineering Division CIMAP, Lucknow HISTORY Written records of herbal distillation are found as early as the first century A.D., and around 1000 A.D., the noted Arab physician and naturalist Ibn Sina also known as Avicenna described the distillation of rose oil from rose petals The ancient Arabian people began to study the chemical properties of essential oils & developed and refined the distillation process Europeans began producing essential oils in the 12th century 1 DISTILLATION ? A process in which a liquid or vapour mixture of two or more substances is separated into its component fractions of desired purity, by the application and removal of heat. In simple terms distillation of aromatic herbs implies vaporizing or liberating the oils from the trichomes / plant cell membranes of the herb in presence of high temperature and moisture and then cooling the vapour mixture to separate out the oil from water. It is the most popular widely used and cost effective method in use today for producing majority of the essential oils throughout the world Distillation is an art and not just a “Chemical" process that is reliant upon many factors for successful quality oil production. BASIC SCIENTIFIC PRINCIPLES INVOLVED IN THE PROCESS To convert any liquid into a vapour we have to apply energy in form of heat called as latent heat of vaporization A liquid always boils at the temperature at which its vapour pressure equals the atmospheric / surrounding pressure For two immiscible liquids the total vapour pressure of the mixture is always equal to the sum of their partial pressures The composition of the mixture will be determined by the concentration of the individual components into its partial pressure As known the boiling point of most essential oil components exceeds that of water and generally lies between 150 – 300oC 2 If a sample of an essential oil having a component ‘A’ having boiling point for example 190oC and the boiling point of the water is 100oC. -

Distillation Accessscience from McgrawHill Education
6/19/2017 Distillation AccessScience from McGrawHill Education (http://www.accessscience.com/) Distillation Article by: King, C. Judson University of California, Berkeley, California. Last updated: 2014 DOI: https://doi.org/10.1036/10978542.201100 (https://doi.org/10.1036/10978542.201100) Content Hide Simple distillations Fractional distillation Vaporliquid equilibria Distillation pressure Molecular distillation Extractive and azeotropic distillation Enhancing energy efficiency Computational methods Stage efficiency Links to Primary Literature Additional Readings A method for separating homogeneous mixtures based upon equilibration of liquid and vapor phases. Substances that differ in volatility appear in different proportions in vapor and liquid phases at equilibrium with one another. Thus, vaporizing part of a volatile liquid produces vapor and liquid products that differ in composition. This outcome constitutes a separation among the components in the original liquid. Through appropriate configurations of repeated vaporliquid contactings, the degree of separation among components differing in volatility can be increased manyfold. See also: Phase equilibrium (/content/phaseequilibrium/505500) Distillation is by far the most common method of separation in the petroleum, natural gas, and petrochemical industries. Its many applications in other industries include air fractionation, solvent recovery and recycling, separation of light isotopes such as hydrogen and deuterium, and production of alcoholic beverages, flavors, fatty acids, and food oils. Simple distillations The two most elementary forms of distillation are a continuous equilibrium distillation and a simple batch distillation (Fig. 1). http://www.accessscience.com/content/distillation/201100 1/10 6/19/2017 Distillation AccessScience from McGrawHill Education Fig. 1 Simple distillations. (a) Continuous equilibrium distillation. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Batch Distillation of Spirits: Experimental Study and Simulation
Research article Received: 5 April 2018 Revised: 11 January 2019 Accepted: 15 January 2019 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jib.560 Batch distillation of spirits: experimental study and simulation of the behaviour of volatile aroma compounds Adrien Douady,1 Cristian Puentes,1 Pierre Awad1,2 and Martine Esteban-Decloux1* This paper focuses on the behaviour of volatile compounds during batch distillation of wine or low wine, in traditional Charentais copper stills, heated with a direct open flame at laboratory (600 L) and industrial (2500 L) scale. Sixty-nine volatile compounds plus ethanol were analysed during the low wine distillation in the 600 L alembic still. Forty-four were quantified and classified according to their concentration profile in the distillate over time and compared with previous studies. Based on the online re- cording of volume flow, density and temperature of the distillate with a Coriolis flowmeter, distillation was simulated with ProSim® BatchColumn software. Twenty-six volatile compounds were taken into account, using the coefficients of the ‘Non- Random Two Liquids’ model. The concentration profiles of 18 compounds were accurately represented, with slight differences in the maximum concentration for seven species together with a single compound that was poorly represented. The distribution of the volatile compounds in the four distillate fractions (heads, heart, seconds and tails) was well estimated by simulation. Fi- nally, data from wine and low wine distillations in the large-scale alembic still (2500 L) were correctly simulated, suggesting that it was possible to adjust the simulation parameters with the Coriolis flowmeter recording and represent the concentration pro- files of most of the quantifiable volatile compounds. -

Distillation of Essential Oils1
WEC310 Distillation of Essential Oils1 Elise V. Pearlstine 2 A short history of essential oils many industries and in new applications as awareness of the benefit of naturally derived products grows. Essential oils are volatile, aromatic oils obtained from plants and used for fragrance, flavoring, and health and beauty applications. Historically, aromatic plants provided important ingredients for perfumes, incense, and cosmetics. They have also been used for ritual purposes and in cooking and medicine. Egyptians used aromatic plant materials to preserve mummies, the Ayurvedic literature of India includes many references to scented substances, ancient Chinese herbalists valued them for their curative properties, and royalty used rare aromatics to perfume themselves and their surroundings. Distillation became an important An eighteenth century still from an old method of obtaining the healing and fragrant Figure 1. monograph by Gildemeister. components of various plants and was well-studied beginning in the 18th and continuing in the 19th Plant anatomy and structure as they centuries (Figure 1). In the 1900s, during the time of relate to essential oil production the industrial revolution, component parts of many essential oils were identified. These components An essential oil is the volatile material derived could then be synthesized for use in perfume and from plant material by a physical process. The plant flavor industries. The art of using essential oils material is usually aromatic and of a single botanical declined during this time but experienced a re-birth in species and form; some essential oil plants have a Europe with aromatherapy later in the century. In different chemical makeup depending on the variety recent years, the use of essential oils has increased in of plant, and the essential oils are correspondingly unique. -

A Guide to Stills & Distilling
A GUIDE TO STILLS & DISTILLING LEADERS IN HOME DISTILLING AND FILTRATION SYSTEMS THAT MEET AND EXCEED EVEN COMMERCIAL STANDARDS. www.stillspirits.com Reorder: 72648 V1 Reorder: With over 25 years in the Air Still A compact, sleek stainless steel still for those who want a no fuss way market, Still Spirits is a leader of producing 1 L (1 US qt) of 40% ABV spirit at a time. Distils alcohol at 60% strength. Used with a small 10 L (2.6 US Gal) fermenter making this in home distilling and filtration system easy to move and operate from any kitchen, boat or campervan. systems that meet and exceed AVERAGE USE even commercial standards. DIFFICULTY Basic CAPACITY 4 L (1 US Gal) The range features: TIME 7- 10 day Fermentation Premium spirits with equivalent commercial quality Approx 2 hours distilling per 1 L (1 US qt) of 40% alcohol at a fraction of the price. YIELD 2 L (2 US qt) of 40% ABV alcohol per 8 L Homemade spirits in as little as 7 days. (2 US Gal) wash Distilled alcohol from a wash made simply using sugar, PURITY Distils at 60% ABV (120 US proof) before being watered down to 40% (80 US proof) yeast, carbon and drinkable water. Homemade spirit flavoured with your choice of Still Spirits spirit or liqueur essences. QUICK GUIDE TO DISTILLING FEATURES WITH THE AIR STILL 1. Wash – Make an 8 L (2 US Gal) wash by • Easy to operate, set up and use. mixing water, sugar, carbon and yeast With no hoses or complicated in the fermenter. -

Dynamic Modelling of Batch Distillation Columns Chemical
Dynamic Modelling of Batch Distillation Columns Maria Nunes de Almeida Viseu Thesis to obtain the Master of Science Degree in Chemical Engineering Supervisors: Prof. Dr Carla Isabel Costa Pinheiro Dr Charles Brand Examination Committee Chairperson: Prof. Dr Sebastião Manuel Tavares da Silva Alves Supervisor: Prof. Dr Carla Isabel Costa Pinheiro Members of the Committee: Prof. Dr João Miguel Alves da Silva November 2014 Page intentionally left blank ii Para os meus pais, Com amor. iii Page intentionally left blank iv Abstract atch distillation is becoming increasingly important in specialty product industries in which flexibility is a B key performance factor. Because high added value chemical compounds are produced in these industries with uncertain demands and lifetimes, mathematical models that predict separation times and product purities thereby facilitating plant scheduling are required. The primary purpose of this study is thus to develop a batch multi-staged distillation model based on mass and energy balances, equilibrium stages and tray hydraulic relations. The mathematical model was implemented in gPROMS ModelBuilder®, an industry- leading custom modelling and flowsheet environment software. Preliminary steps were undertaken prior to implementing the dynamic multi-staged model: batch distillation operating policies as well as modelling and tray hydraulic considerations were covered in a broad background review; a theoretical separation example of an equimolar benzene/toluene mixture was used to validate a simpler Rayleigh distillation model comprising only one equilibrium stage; tray hydraulic correlations encompassing column diameter, tray holdup and tray pressure drop estimations were tested in a methanol/water continuous separation case study. The multi-staged batch model was validated for a methanol/water separation using literature data from an experimental pilot plant and from theoretical results given by a model implemented in Fortran language and by commercial simulator Batchsim of Pro/II.