Wood Decay Fungi in Landscape Trees
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Field Guide to Common Macrofungi in Eastern Forests and Their Ecosystem Functions
United States Department of Field Guide to Agriculture Common Macrofungi Forest Service in Eastern Forests Northern Research Station and Their Ecosystem General Technical Report NRS-79 Functions Michael E. Ostry Neil A. Anderson Joseph G. O’Brien Cover Photos Front: Morel, Morchella esculenta. Photo by Neil A. Anderson, University of Minnesota. Back: Bear’s Head Tooth, Hericium coralloides. Photo by Michael E. Ostry, U.S. Forest Service. The Authors MICHAEL E. OSTRY, research plant pathologist, U.S. Forest Service, Northern Research Station, St. Paul, MN NEIL A. ANDERSON, professor emeritus, University of Minnesota, Department of Plant Pathology, St. Paul, MN JOSEPH G. O’BRIEN, plant pathologist, U.S. Forest Service, Forest Health Protection, St. Paul, MN Manuscript received for publication 23 April 2010 Published by: For additional copies: U.S. FOREST SERVICE U.S. Forest Service 11 CAMPUS BLVD SUITE 200 Publications Distribution NEWTOWN SQUARE PA 19073 359 Main Road Delaware, OH 43015-8640 April 2011 Fax: (740)368-0152 Visit our homepage at: http://www.nrs.fs.fed.us/ CONTENTS Introduction: About this Guide 1 Mushroom Basics 2 Aspen-Birch Ecosystem Mycorrhizal On the ground associated with tree roots Fly Agaric Amanita muscaria 8 Destroying Angel Amanita virosa, A. verna, A. bisporigera 9 The Omnipresent Laccaria Laccaria bicolor 10 Aspen Bolete Leccinum aurantiacum, L. insigne 11 Birch Bolete Leccinum scabrum 12 Saprophytic Litter and Wood Decay On wood Oyster Mushroom Pleurotus populinus (P. ostreatus) 13 Artist’s Conk Ganoderma applanatum -

Bridgeoporus Nobilissimus Is Much More Abundant Than Indicated by the Presence of Basidiocarps in Forest Stands
North American Fungi Volume 10, Number 3, Pages 1-28 Published May 29, 2015 Bridgeoporus nobilissimus is much more abundant than indicated by the presence of basidiocarps in forest stands Matthew Gordon1 and Kelli Van Norman2 1Molecular Solutions LLC, 715 NW Hoyt St., #2546, Portland, OR 97208, USA 2Interagency Special Status/Sensitive Species Program, USDI Bureau of Land Management Oregon State Office & USDA Forest Service Region 6, 1220 SW 3rd Ave., Portland, OR 97204, USA Gordon, M., and K. Van Norman. 2015. Bridgeoporus nobilissimus is much more abundant than indicated by the presence of basidiocarps in forest stands. North American Fungi 10(3): 1-28. http://dx.doi:10.2509/naf2015.010.003 Corresponding author: Matt Gordon [email protected]. Accepted for publication May 4, 2015. http://pnwfungi.org Copyright © 2015 Pacific Northwest Fungi Project. All rights reserved. Abstract: The polypore Bridgeoporus nobilissimus produces large perennial basidiocarps on large diameter Abies stumps, snags and trees in coniferous forests of the Pacific Northwest. Despite the size and persistence of the basidiocarps, they are rarely observed, making the conservation of this species a concern. We determined that a genetic marker for this fungus could be detected in DNA extracted from wood cores taken from trees hosting basidiocarps. We then tested 105 trees and stumps that did not host B. nobilissimus basidiocarps in plots surrounding B. nobilissimus conks, and 291 trees and stumps in randomly located plots in four stands that contained at least one B. nobilissimus basidiocarp. We found that trees of all sizes throughout all of the stands hosted B. -

A New Record of Ganoderma Tropicum (Basidiomycota, Polyporales) for Thailand and First Assessment of Optimum Conditions for Mycelia Production
A peer-reviewed open-access journal MycoKeys 51:A new65–83 record (2019) of Ganoderma tropicum (Basidiomycota, Polyporales) for Thailand... 65 doi: 10.3897/mycokeys.51.33513 RESEARCH ARTICLE MycoKeys http://mycokeys.pensoft.net Launched to accelerate biodiversity research A new record of Ganoderma tropicum (Basidiomycota, Polyporales) for Thailand and first assessment of optimum conditions for mycelia production Thatsanee Luangharn1,2,3,4, Samantha C. Karunarathna1,3,4, Peter E. Mortimer1,4, Kevin D. Hyde3,5, Naritsada Thongklang5, Jianchu Xu1,3,4 1 Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China 2 University of Chinese Academy of Sciences, Bei- jing 100049, China 3 East and Central Asia Regional Office, World Agroforestry Centre (ICRAF), Kunming 650201, Yunnan, China 4 Centre for Mountain Ecosystem Studies (CMES), Kunming Institute of Botany, Kunming 650201, Yunnan, China 5 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand Corresponding author: Jianchu Xu ([email protected]); Peter E. Mortimer ([email protected]) Academic editor: María P. Martín | Received 30 January 2019 | Accepted 12 March 2019 | Published 7 May 2019 Citation: Luangharn T, Karunarathna SC, Mortimer PE, Hyde KD, Thongklang N, Xu J (2019) A new record of Ganoderma tropicum (Basidiomycota, Polyporales) for Thailand and first assessment of optimum conditions for mycelia production. MycoKeys 51: 65–83. https://doi.org/10.3897/mycokeys.51.33513 Abstract In this study a new record of Ganoderma tropicum is described as from Chiang Rai Province, Thailand. The fruiting body was collected on the base of a livingDipterocarpus tree. -

Identification, Effects and Management of 5 Types of Decay Organisms Found in Seattle Parks
Identification, Effects and Management of 5 types of decay organisms found in Seattle Parks Chris Rippey, Arborist [email protected] • Third generation Arborist • Grew up in the bay area of California. • Was 16 when I started working with my dad in tree care • I fell in love with tree work, not trees • Managed the preventative tree maintenance programs at Stanford University for 14 years. • Moved to Washington and began working for Seattle Parks 2 ½ years ago Seattle Parks System - 6,412 Total Acres - 4,016 Acres of Developed Park - 2,396 Acres of Natural Area - 480 Parks - >300,000 trees - >16,000 trees in our tree inventory Seward Park 1920 Ravenna Park 1922 What are we focusing on? - 171,615 trees in our Buffer Zone. - Buffer Zone is a 50’ buffer around high use areas like beaches, paved roads and trails, playgrounds…etc) - Buffer Zones are on average 56% of a given park Tree Risk Inspections Terms & Matrix TERM DEFINITION Likelihood of failure and impacts Imminent Failure has started or is most likely to occur in the near future even if there is no weather forces/rare occurrence. Will fail in a storm. Probable Failure may be expected under normal weather within a time frame. Likely to fail in a severe storm. Possible Failure could occur, but is unlikely during normal weather. May fail in a severe storm. Improbable Tree or branch failure not likely under normal conditions and may not fail in severe weather within a time frame. Risk rating High Failed tree or part will likely impact a target. -

Wood-Inhabiting Fungi in Southern China 2. Polypores from Sichuan Province
Ann. Bot. Fennici 41: 319–329 ISSN 0003-3847 Helsinki 19 October 2004 © Finnish Zoological and Botanical Publishing Board 2004 Wood-inhabiting fungi in southern China 2. Polypores from Sichuan Province Yu-Cheng Dai, Yu-Lian Wei & Zheng Wang Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China Received 20 Jan. 2004, revised version received 9 Apr. 2004, accepted 16 Apr. 2004 Dai, Y. C., Wei, Y. L. & Wang, Z. 2004: Wood-inhabiting fungi in southern China 2. Polypores from Sichuan Province. — Ann. Bot. Fennici 41: 319–329. 250 specimens of polypores were collected in the Jiuzhaigou and Huanglong nature reserves, and the Qingcheng and Ermei Mountains in Sichuan Province of southwest- ern China in October 2002. Of the 132 poroid species identified, 92 are here reported for the first time from Sichuan, and 14 are new to China. Two new species, Mega- sporoporia rhododendri Y.C. Dai & Y.L. Wei and Oxyporus macroporus Y.C. Dai & Y.L. Wei are described and illustrated..The first-mentioned species is characterized by resupinate or rarely effused-reflexed basidiocarps with pale greyish cream pore sur- face, large and ellipsoid basidiospores, and by its living exclusively on Rhododendron. Oxyporus macroporus is distinguished from the other species in the genus by its exten- sive basidiocarps, perennial habit, large pores, and by its occurrence on coniferous hosts. A new combination, Haploporus nepalensis (T. Hattori) Y.C. Dai is proposed. A checklist of Sichuan polypores, including their substrates and collecting data, is provided, based on our own materials. The major component of the northern Sich- uan polypore flora are widely distributed circumpolar, temperate and boreal species, found at higher elevations in the two nature reserves. -

Diseases of Trees in the Great Plains
39. Ganoderma Root Rot or White Mottled Rot James T. Blodgett Ganoderma root rot, also called white mottled rot, is caused by the fungus Ganoderma applanatum. This fungus is found in all 50 states and occurs throughout North America and Europe. It is a pathogen and a common wood-decaying fungus of roots and lower stems (butts) of many deciduous and some coniferous trees species. Ganoderma-caused root rot has been reported in live trees, such as apple (Malus spp.), aspen (Populus spp.), basswood (Tilia spp.), beech (Fagus spp.), birch (Betula spp.), cherry (Prunus spp.), citrus (Citrus spp.), cottonwood (Populus spp.), elm (Ulmus spp.), hemlock (Tsuga spp.), hornbeam (Carpinus caroliniana), horsechestnut (Aesculus hippocastanum), black locust (Robinia pseudoacacia) and honeylocust (Gleditsia triacanthos), maple (Acer spp.), mul- berry (Morus spp.), oak (Quercus spp.), spruce (Picea spp.), sycamore (Platanus occidentalis), tulip tree (Liriodendron tulipifera), sweetgum (Liquidambar styraciflua), and willow (Salix spp.). G. applanatum is commonly known as the artist’s conk. The name comes from the use of its fruiting bodies as a drawing medium by artists (fig. 39-1). When the fresh lower surface is rubbed or scratched, it immediately changes Figure 39-1—Drawing on the lower from white to dark brown, producing shading or surface of Ganoderma applanatum conk visible lines. When the conk is dried, drawings (James J. Worrall, U.S. Forest Service). become permanent. Hosts and Distribution In the Great Plains, G. applanatum occurs predominantly in aspen, cottonwoods, and other Populus species. This fungal pathogen is irregularly distributed across the Great Plains, but its distribution is not well documented in many areas. -

Antibacterial Activity, Optimal Culture Conditions and Cultivation of the Medicinal Ganoderma Australe, New to Thailand
1108 Mycosphere 8(8): 1108–1123 (2017) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/8/8/11 Copyright © Guizhou Academy of Agricultural Sciences Antibacterial activity, optimal culture conditions and cultivation of the medicinal Ganoderma australe, new to Thailand Luangharn T1,2, Karunarathna SC1,2, Khan S1,2, Xu JC1,2*, Mortimer PE1,2 and Hyde KD1,2,3,4,5 1 Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming 650201, China. 2 World Agroforestry Centre, China & East-Asia Office, 132 Lanhei Road, Kunming 650201, China.3 3 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand. 4 School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand. 5 Mushroom Research Foundation, 128 M.3 Ban Pa Deng T. Pa Pae, A. Mae Taeng, Chiang Mai 50150, Thailand. Luangharn T, Karunarathna SC, Khan S, Xu JC, Mortimer PE, Hyde KD 2017 – Antibacterial activity, optimal culture conditions and cultivation of the medicinal Ganoderma australe, new to Thailand. Mycosphere 8(8), 1108–1123, Doi 10.5943/mycosphere/8/8/11 Abstract Ganoderma is a well-known genus of medicinal mushrooms that belongs to the order Polyporales. Many members of this genus are extensively used in traditional Asian medicines. Herein we report a new strain of Ganoderma australe collected in Thailand and identified using macro- and micro-morphological characteristics as well as phylogenetic analysis. The optimal conditions for mycelia growth were 25–30 ºC at pH 7–8, while sorghum and barley were found to be the best grain media for spawn production. -

A Higher-Level Phylogenetic Classification of the Fungi
mycological research 111 (2007) 509–547 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/mycres A higher-level phylogenetic classification of the Fungi David S. HIBBETTa,*, Manfred BINDERa, Joseph F. BISCHOFFb, Meredith BLACKWELLc, Paul F. CANNONd, Ove E. ERIKSSONe, Sabine HUHNDORFf, Timothy JAMESg, Paul M. KIRKd, Robert LU¨ CKINGf, H. THORSTEN LUMBSCHf, Franc¸ois LUTZONIg, P. Brandon MATHENYa, David J. MCLAUGHLINh, Martha J. POWELLi, Scott REDHEAD j, Conrad L. SCHOCHk, Joseph W. SPATAFORAk, Joost A. STALPERSl, Rytas VILGALYSg, M. Catherine AIMEm, Andre´ APTROOTn, Robert BAUERo, Dominik BEGEROWp, Gerald L. BENNYq, Lisa A. CASTLEBURYm, Pedro W. CROUSl, Yu-Cheng DAIr, Walter GAMSl, David M. GEISERs, Gareth W. GRIFFITHt,Ce´cile GUEIDANg, David L. HAWKSWORTHu, Geir HESTMARKv, Kentaro HOSAKAw, Richard A. HUMBERx, Kevin D. HYDEy, Joseph E. IRONSIDEt, Urmas KO˜ LJALGz, Cletus P. KURTZMANaa, Karl-Henrik LARSSONab, Robert LICHTWARDTac, Joyce LONGCOREad, Jolanta MIA˛ DLIKOWSKAg, Andrew MILLERae, Jean-Marc MONCALVOaf, Sharon MOZLEY-STANDRIDGEag, Franz OBERWINKLERo, Erast PARMASTOah, Vale´rie REEBg, Jack D. ROGERSai, Claude ROUXaj, Leif RYVARDENak, Jose´ Paulo SAMPAIOal, Arthur SCHU¨ ßLERam, Junta SUGIYAMAan, R. Greg THORNao, Leif TIBELLap, Wendy A. UNTEREINERaq, Christopher WALKERar, Zheng WANGa, Alex WEIRas, Michael WEISSo, Merlin M. WHITEat, Katarina WINKAe, Yi-Jian YAOau, Ning ZHANGav aBiology Department, Clark University, Worcester, MA 01610, USA bNational Library of Medicine, National Center for Biotechnology Information, -

MUSHROOMS of the OTTAWA NATIONAL FOREST Compiled By
MUSHROOMS OF THE OTTAWA NATIONAL FOREST Compiled by Dana L. Richter, School of Forest Resources and Environmental Science, Michigan Technological University, Houghton, MI for Ottawa National Forest, Ironwood, MI March, 2011 Introduction There are many thousands of fungi in the Ottawa National Forest filling every possible niche imaginable. A remarkable feature of the fungi is that they are ubiquitous! The mushroom is the large spore-producing structure made by certain fungi. Only a relatively small number of all the fungi in the Ottawa forest ecosystem make mushrooms. Some are distinctive and easily identifiable, while others are cryptic and require microscopic and chemical analyses to accurately name. This is a list of some of the most common and obvious mushrooms that can be found in the Ottawa National Forest, including a few that are uncommon or relatively rare. The mushrooms considered here are within the phyla Ascomycetes – the morel and cup fungi, and Basidiomycetes – the toadstool and shelf-like fungi. There are perhaps 2000 to 3000 mushrooms in the Ottawa, and this is simply a guess, since many species have yet to be discovered or named. This number is based on lists of fungi compiled in areas such as the Huron Mountains of northern Michigan (Richter 2008) and in the state of Wisconsin (Parker 2006). The list contains 227 species from several authoritative sources and from the author’s experience teaching, studying and collecting mushrooms in the northern Great Lakes States for the past thirty years. Although comments on edibility of certain species are given, the author neither endorses nor encourages the eating of wild mushrooms except with extreme caution and with the awareness that some mushrooms may cause life-threatening illness or even death. -

Collecting and Recording Fungi
British Mycological Society Recording Network Guidance Notes COLLECTING AND RECORDING FUNGI A revision of the Guide to Recording Fungi previously issued (1994) in the BMS Guides for the Amateur Mycologist series. Edited by Richard Iliffe June 2004 (updated August 2006) © British Mycological Society 2006 Table of contents Foreword 2 Introduction 3 Recording 4 Collecting fungi 4 Access to foray sites and the country code 5 Spore prints 6 Field books 7 Index cards 7 Computers 8 Foray Record Sheets 9 Literature for the identification of fungi 9 Help with identification 9 Drying specimens for a herbarium 10 Taxonomy and nomenclature 12 Recent changes in plant taxonomy 12 Recent changes in fungal taxonomy 13 Orders of fungi 14 Nomenclature 15 Synonymy 16 Morph 16 The spore stages of rust fungi 17 A brief history of fungus recording 19 The BMS Fungal Records Database (BMSFRD) 20 Field definitions 20 Entering records in BMSFRD format 22 Locality 22 Associated organism, substrate and ecosystem 22 Ecosystem descriptors 23 Recommended terms for the substrate field 23 Fungi on dung 24 Examples of database field entries 24 Doubtful identifications 25 MycoRec 25 Recording using other programs 25 Manuscript or typescript records 26 Sending records electronically 26 Saving and back-up 27 Viruses 28 Making data available - Intellectual property rights 28 APPENDICES 1 Other relevant publications 30 2 BMS foray record sheet 31 3 NCC ecosystem codes 32 4 Table of orders of fungi 34 5 Herbaria in UK and Europe 35 6 Help with identification 36 7 Useful contacts 39 8 List of Fungus Recording Groups 40 9 BMS Keys – list of contents 42 10 The BMS website 43 11 Copyright licence form 45 12 Guidelines for field mycologists: the practical interpretation of Section 21 of the Drugs Act 2005 46 1 Foreword In June 2000 the British Mycological Society Recording Network (BMSRN), as it is now known, held its Annual Group Leaders’ Meeting at Littledean, Gloucestershire. -

Ohio Plant Disease Index
Special Circular 128 December 1989 Ohio Plant Disease Index The Ohio State University Ohio Agricultural Research and Development Center Wooster, Ohio This page intentionally blank. Special Circular 128 December 1989 Ohio Plant Disease Index C. Wayne Ellett Department of Plant Pathology The Ohio State University Columbus, Ohio T · H · E OHIO ISJATE ! UNIVERSITY OARilL Kirklyn M. Kerr Director The Ohio State University Ohio Agricultural Research and Development Center Wooster, Ohio All publications of the Ohio Agricultural Research and Development Center are available to all potential dientele on a nondiscriminatory basis without regard to race, color, creed, religion, sexual orientation, national origin, sex, age, handicap, or Vietnam-era veteran status. 12-89-750 This page intentionally blank. Foreword The Ohio Plant Disease Index is the first step in develop Prof. Ellett has had considerable experience in the ing an authoritative and comprehensive compilation of plant diagnosis of Ohio plant diseases, and his scholarly approach diseases known to occur in the state of Ohia Prof. C. Wayne in preparing the index received the acclaim and support .of Ellett had worked diligently on the preparation of the first the plant pathology faculty at The Ohio State University. edition of the Ohio Plant Disease Index since his retirement This first edition stands as a remarkable ad substantial con as Professor Emeritus in 1981. The magnitude of the task tribution by Prof. Ellett. The index will serve us well as the is illustrated by the cataloguing of more than 3,600 entries complete reference for Ohio for many years to come. of recorded diseases on approximately 1,230 host or plant species in 124 families. -
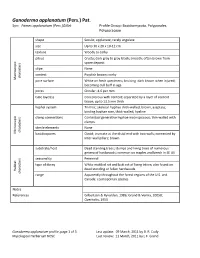
Ganoderma Applanatum (Pers.) Pat
Ganoderma applanatum (Pers.) Pat. Syn: Fomes applanatum (Pers.)Gillet Profile Group: Basidiomycota, Polyporales, Polyporaceae shape Sessile; applanate; rarely ungulate size Up to 30 x 20 x 10-12 cm texture Woody to corky pileus Crusty; dark gray to gray-black; smooth; often brown from spore deposit stipe None context Purplish brown; corky characters Macroscopic pore surface White on fresh specimens; bruising dark brown when injured; becoming dull buff in age pores Circular; 4-6 per mm tube layer(s) Concolorous with context; separated by a layer of context tissue; up to 13.5 mm thick hyphal system Trimitic; skeletal hyphae thick-walled, brown, aseptate; binding hyphae rare, thick-walled, hyaline clamp connections Contextual generative hyphae inconspicuous; thin-walled with clamps sterile elements None characters Microscopic basidiospores Ovoid; truncate at the distal end with two walls; connected by inter-wall pillars; brown substrate/host Dead standing trees; stumps and living trees of numerous genera of hardwoods; common on maples and beech in SE US seasonality Perennial type of decay White mottled rot and butt rot of living trtees; also found on dead standing or fallen hardwoods Habitat characters range Apparently throughout the forest regions of the U.S. and Canada; cosmopolitan species Notes References Gilbertson & Ryvarden, 1986; Grand & Vernia, 2005B; Overholts, 1953 Ganoderma applanatum profile, page 1 of 3 Last update: 09 March, 2011 by B. R. Cody Mycological Herbarium NCSC Last review: 11 March, 2011 by L.F. Grand Species distribution in North Carolina Ganoderma applanatum (Pers.) Pat. Habit of Basidiocarps Habit of Basidiocarps Habit of Basidiocarp Habit of Basidiocarp Ganoderma applanatum profile, page 2 of 3 Last update: 09 March, 2011 by B.