Microglia-Mediated Neurodegeneration in Perinatal Brain Injuries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Clinical Neurophysiology Board Review Q&A
Clinical Neurophysiology Board Review Board Clinical Neurophysiology Clinical Neurophysiology Board Review Q&A Clinical Puneet K. Gupta, MD, MSE • Pradeep N. Modur, MD, MS • Srikanth Muppidi, MD his high-yield, illustrated clinical neurophysiology board review is a comprehen- Neurophysiology sive resource for assessing and refining the knowledge tested on multiple board Texaminations. Written by authors who are collectively board certified in all of the areas covered, the book is a valuable study tool for candidates preparing for certifica- tion or recertification in clinical neurophysiology, neuromuscular medicine, epilepsy, Board Review sleep medicine, and neurology. Using structured question formats typically encountered on boards, this comprehensive review allows users to assess their knowledge in a wide range of topics, provides rationales for correct answers, and explains why the other choices are incorrect. A unique “Pearls” section at the end of the book allows for quick review of the most important concepts prior to exam day. Clinical Neurophysiology Board Review Q&A contains 801 questions with answers and detailed explanations. The book is divided into eight chapters covering anatomy Q and physiology, electronics and instrumentation, nerve conduction studies and EMG, & EEG, evoked potentials and intraoperative monitoring, sleep studies, ethics and safety, and advanced topics including QEEG, MEG, TES, autonomic testing, and more. A Liberal use of image-based questions illustrating the full spectrum of neurophysiologic & tests and findings build interpretive skills. Questions are randomized and include Q A both case-related questions in series and stand-alone items to familiarize candidates Gu with the question types and formats they will find on the exam. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

NF-Jb Signalling Requirement for Brain Myelin Formation Is Shown by Genotype/MRI Phenotype Correlations in Patients with Xq28 Duplications
European Journal of Human Genetics (2013) 21, 195–199 & 2013 Macmillan Publishers Limited All rights reserved 1018-4813/13 www.nature.com/ejhg ARTICLE NF-jB signalling requirement for brain myelin formation is shown by genotype/MRI phenotype correlations in patients with Xq28 duplications Orianne Philippe1, Marle`ne Rio1, Vale´rie Malan1, Hilde Van Esch2, Genevie`ve Baujat1, Nadia Bahi-Buisson3, Vassili Valayannopoulos4, Roseline Gesny1, Jean-Paul Bonnefont1, Arnold Munnich1,GuyFroyen5, Jeanne Amiel1, Nathalie Boddaert1,3 and Laurence Colleaux*,1 One of the key signals regulating peripheral myelin formation by Schwann cell is the activation of the transcription factor NF-jB. Yet, whether NF-jB exerts similar functions in central myelin formation by oligodendrocytes remains largely unknown. We previously reported white matter abnormalities with unusual discordance between T2 and FLAIR sequences in a patient with intellectual disability and defective NF-jB signalling. These observations prompted us to hypothesise that NF-jB signalling may have a role in the axon myelination process of central neurons. We report here on five male patients with Xq28 duplications encompassing MECP2, three of which presented white matter anomalies on brain MRI. Array-CGH and FISH analyses demonstrated that brain abnormalities correlate with additional copies of the IKBKG, a gene encoding a key regulator of NF-jB activation. Quantitative RT-PCR experiments and jB-responsive reporter gene assays provide evidence that IKBKG overexpression causes impaired NF-jB signalling in skin fibroblasts derived from patients with white matter anomalies. These data further support the role of NF-jB signalling in astroglial cells for normal myelin formation of the central nervous system. -

Integrating Brain-Based Psychoeducation Into Clinical Practice Raissa Miller Boise State University
Boise State University ScholarWorks Counselor Education Faculty Publications and Department of Counselor Education Presentations 4-1-2016 Neuroeducation: Integrating Brain-Based Psychoeducation into Clinical Practice Raissa Miller Boise State University This document was originally published in Journal of Mental Health Counseling by the American Mental Health Counselors Association. Copyright restrictions may apply. doi: 10.17744/mehc.38.2.02 Volume 38/Number 2/April 2016/Pages 103-1 IS/doi: 10. l7744/mehc.38.2.02 PRACTICE Neuroeducation: Integrating Brain- Based Psychoeducation into Clinical Practice Raissa M iller Boise State University Understanding and integrating neuroscience research into clinical practice represents a rapidly growing area in mental health. An expanding body of neuroscience literature increasingly informs clinical practice by validating theory, guiding clinical assessment and conceptualiza tion, directing effective interventions, and facilitating cross-disciplinary communication. Little attention, however, has been given to the use of neuroeducation with clients. In this article, the author provides mental health counselors with a definition of neuroeducation and a rationale for incorporating neuroeducation into clinical practice. The author identifies common neuro education topics and offers activity suggestions to illustrate their use in counseling. Finally, the author offers best practices for implementing neuroeducation, including attention to counselor competence, client readiness, and neuroscience of learning -

Supplementation with Complex Milk Lipids During Brain Development Promotes Neuroplasticity Without Altering Myelination Or Vascular Density
food & nutrition æ research ORIGINAL ARTICLE Supplementation with complex milk lipids during brain development promotes neuroplasticity without altering myelination or vascular density Rosamond B. Guillermo1,2, Panzao Yang1,2, Mark H. Vickers1, Paul McJarrow3 and Jian Guan1,2* 1Liggins Institute, The University of Auckland, Auckland, New Zealand; 2Centre for Brain Research, Faculty of Medical and Health Sciences, The University of Auckland, Auckland, New Zealand; 3Fonterra Research and Development Centre, Palmerston North, New Zealand Abstract Background: Supplementation with complex milk lipids (CML) during postnatal brain development has been shown to improve spatial reference learning in rats. Objective: The current study examined histo-biological changes in the brain following CML supplementation and their relationship to the observed improvements in memory. Design: The study used the brain tissues from the rats (male Wistar, 80 days of age) after supplementing with either CML or vehicle during postnatal day 10Á80. Immunohistochemical staining of synaptophysin, glutamate receptor-1, myelin basic protein, isolectin B-4, and glial fibrillary acidic protein was performed. The average area and the density of the staining and the numbers of astrocytes and capillaries were assessed and analysed. Results: Compared with control rats, CML supplementation increased the average area of synaptophysin staining and the number of GFAP astrocytes in the CA3 sub-region of the hippocampus (pB0.01), but not in the CA4 sub-region. The supplementation also led to an increase in dopamine output in the striatum that was related to nigral dopamine expression (pB0.05), but did not alter glutamate receptors, myelination or vascular density. Conclusion: CML supplementation may enhance neuroplasticity in the CA3 sub-regions of the hippocampus. -
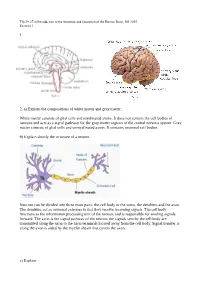
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Iron Deficiency Alters Auditory Recognition Memory in Newborn
0031-3998/04/5506-1034 PEDIATRIC RESEARCH Vol. 55, No. 6, 2004 Copyright © 2004 International Pediatric Research Foundation, Inc. Printed in U.S.A. Iron Deficiency Alters Auditory Recognition Memory in Newborn Infants of Diabetic Mothers ASHAJYOTHI M. SIDDAPPA, MICHAEL K. GEORGIEFF, SANDI WEWERKA, CATHY WORWA, CHARLES A. NELSON, AND RAYE-ANN DEREGNIER Division of Neonatology, Department of Pediatrics [A.M.S., M.K.G., C.A.N.], Institute of Child Development [M.K.G., S.W., C.A.N.], and Center for Neurobehavioral Development [M.K.G., S.W., C.A.N.], University of Minnesota, Minneapolis, MN 55455, U.S.A.; Division of Neonatology [C.W.], Children’s Hospital-St. Paul, St. Paul, MN 55102, U.S.A.; and Division of Neonatology, Department of Pediatrics [R.-A.d.R.], Northwestern University Feinberg School of Medicine & Children’s Memorial Hospital, Chicago, IL 60611, U.S.A. ABSTRACT Infants of diabetic mothers (IDMs) are at risk for perinatal voices compared with the mothers’ voices, whereas the BID brain iron deficiency that may target the developing hippocam- group did not. Higher cord ferritin concentrations were correlated pus. The objective of this study was to evaluate hippocampally with larger negative slow waves at the right temporal (T4) based recognition memory and infant development in IDMs with electrode site. At1yofage, motor development was slower in suspected brain iron deficiency (BID; cord ferritin Յ34 g/L) the BID group than in the BIS group. IDMs suspected to have compared with IDMs with sufficient brain iron stores (BIS; cord BID demonstrated impaired neonatal auditory recognition mem- ferritin Ͼ34 g/L) using event-related potentials (ERPs). -

Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons
The Journal of Neuroscience, September 1989, g(9): 30303039 Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons Gilles Laurent and Malcolm Burrows Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, England The gain of local reflexes of one leg of a locust can be altered partmentalized, with synaptic inputs and their associated by mechanosensory inputs generated by movements of or conductance changes restricted to particular branches. In tactile inputs to an adjacent leg. Touching the mesothoracic this way, an individual nonspiking neuron could contribute tarsus, for example, increases the number of spikes that are simultaneously to several local circuits. The inputs from dif- produced by the metathoracic slow extensor tibiae motor ferent intersegmental interneurons could then modulate these neuron and enhances the depolarization of flexor tibiae mo- pathways independently. tor neuron in response to imposed movements of the chor- dotonal organ in the ipsilateral hind femur. The sensory in- Nonspiking interneurons in the metathoracic ganglion of the formation from the middle leg is conveyed directly to locust receive direct inputs from mechanosensory afferents on nonspiking interneurons and motor neurons controlling the one hindleg and are essential elements in local reflex movements movements of the hindleg by a population of mesothoracic of that leg (Laurent and Burrows, 1988; Burrows et al., 1988). intersegmental interneurons (Laurent and Burrows, 1989). They also receive direct inputs from intersegmental intemeu- The metathoracic nonspiking interneurons receive direct in- rons that process the mechanosensory inputs from an ipsilateral puts from receptors on a hindleg and are, therefore, a point middle leg (Laurent and Burrows, 1989).