Modeling Food Webs: Exploring Unexplained Structure Using Latent Traits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dna Sequence Data Indicates the Polyphyl Y of the Family Ctenidae (Araneae )
1993. The Journal of Arachnology 21 :194–201 DNA SEQUENCE DATA INDICATES THE POLYPHYL Y OF THE FAMILY CTENIDAE (ARANEAE ) Kathrin C . Huber', Thomas S . Haider2, Manfred W . Miiller2, Bernhard A . Huber' , Rudolf J. Schweyen2, and Friedrich G . Barth' : 'Institut fair Zoologie, Althanstr . 14; 1090 Wien; and 2lnstitut fur Mikrobiologie and Genetik; Dr. Bohrgasse 9 ; 1030 Wien (Vienna), Austria . ABSTRACT. Mitochondrial DNA fragments comprising more than 400 bases of the 16S rDNA from nine spider species have been sequenced: Cupiennius salei, C. getazi, C. coccineus and Phoneutria boliviensis (Ctenidae), Pisaura mirabilis, Dolomedes fimbriatus (Pisauridae), Pardosa agrestis (Lycosidae), Clubiona pallidula (Clubi- onidae) and Ryuthela nishihirai (syn. Heptathela nishihirai; Heptathelidae: Mesothelae). Sequence divergence ranges from 3–4% among Cupiennius species and up to 36% in pairwise comparisons of the more distantly related spider DNAs. Maximally parsimonious gene trees based on these sequences indicate that Phoneutri a and Cupiennius are the most distantly related species of the examined Lycosoidea . The monophyly of the family Ctenidae is therefore doubted ; and a revision of the family, which should include DNA-data, is needed . Cupiennius salei (Ctenidae) is one of the most get a high copy number of the DNA segment of extensively studied species of spiders (see Lach - interest. The PCR depends on the availability of muth et al. 1985). The phylogeny of the Ctenidae , oligonucleotides that specifically bind to the a mainly South and Central American family, i s flanking sequences of this DNA segment. These poorly understood ; and systematists propose oligonucleotides serve as primers for a polymer- highly contradicting views on its classification ization reaction that copies the segment in vitro. -

F4 URS Protected Species Report 2012
Homes and Communities Agency Northstowe Phase 2 Environmental Statement F4 URS Protected Species Report 2012 Page F4 | | Northstowe Protected Species Report July 2013 47062979 Prepared for: Homes and Communities Agency UNITED KINGDOM & IRELAND REVISION RECORD Rev Date Details Prepared by Reviewed by Approved by 2 December Version 2 Rachel Holmes Steve Muddiman Steve Muddiman 2012 Principal Associate Associate Environmental Ecologist Ecologist Consultant 4 January Version 4 Rachel Holmes Steve Muddiman Steve Muddiman 2013 Principal Associate Associate Environmental Ecologist Ecologist Consultant 5 July 2013 Version 5 Rachel Holmes Steve Muddiman Steve Muddiman Principal Associate Associate Environmental Ecologist Ecologist Consultant URS Infrastructure & Environment UK Limited St George’s House, 5 St George’s Road Wimbledon, London SW19 4DR United Kingdom PROTECTED SPECIES REPORT 2 2013 Limitations URS Infrastructure & Environment UK Limited (“URS”) has prepared this Report for the sole use of Homes and Communities Agency (“Client”) in accordance with the Agreement under which our services were performed (proposal dated 7th April and 23 rd July 2012). No other warranty, expressed or implied, is made as to the professional advice included in this Report or any other services provided by URS. This Report is confidential and may not be disclosed by the Client nor relied upon by any other party without the prior and express written agreement of URS. The conclusions and recommendations contained in this Report are based upon information provided by others and upon the assumption that all relevant information has been provided by those parties from whom it has been requested and that such information is accurate. Information obtained by URS has not been independently verified by URS, unless otherwise stated in the Report. -

Insecta Zeitschrift Für Entomologie Und Naturschutz
Insecta Zeitschrift für Entomologie und Naturschutz Heft 9/2004 Insecta Bundesfachausschuss Entomologie Zeitschrift für Entomologie und Naturschutz Heft 9/2004 Impressum © 2005 NABU – Naturschutzbund Deutschland e.V. Herausgeber: NABU-Bundesfachausschuss Entomologie Schriftleiter: Dr. JÜRGEN DECKERT Museum für Naturkunde der Humbolt-Universität zu Berlin Institut für Systematische Zoologie Invalidenstraße 43 10115 Berlin E-Mail: [email protected] Redaktion: Dr. JÜRGEN DECKERT, Berlin Dr. REINHARD GAEDIKE, Eberswalde JOACHIM SCHULZE, Berlin Verlag: NABU Postanschrift: NABU, 53223 Bonn Telefon: 0228.40 36-0 Telefax: 0228.40 36-200 E-Mail: [email protected] Internet: www.NABU.de Titelbild: Die Kastanienminiermotte Cameraria ohridella (Foto: J. DECKERT) siehe Beitrag ab Seite 9. Gesamtherstellung: Satz- und Druckprojekte TEXTART Verlag, ERIK PIECK, Postfach 42 03 11, 42403 Solingen; Wolfsfeld 12, 42659 Solingen, Telefon 0212.43343 E-Mail: [email protected] Insecta erscheint in etwa jährlichen Abständen ISSN 1431-9721 Insecta, Heft 9, 2004 Inhalt Vorwort . .5 SCHULZE, W. „Nachbar Natur – Insekten im Siedlungsbereich des Menschen“ Workshop des BFA Entomologie in Greifswald (11.-13. April 2003) . .7 HOFFMANN, H.-J. Insekten als Neozoen in der Stadt . .9 FLÜGEL, H.-J. Bienen in der Großstadt . .21 SPRICK, P. Zum vermeintlichen Nutzen von Insektenkillerlampen . .27 MARTSCHEI, T. Wanzen (Heteroptera) als Indikatoren des Lebensraumtyps Trockenheide in unterschiedlichen Altersphasen am Beispiel der „Retzower Heide“ (Brandenburg) . .35 MARTSCHEI, T., Checkliste der bis jetzt bekannten Wanzenarten H. D. ENGELMANN Mecklenburg-Vorpommerns . .49 DECKERT, J. Zum Vorkommen von Oxycareninae (Heteroptera, Lygaeidae) in Berlin und Brandenburg . .67 LEHMANN, U. Die Bedeutung alter Funddaten für die aktuelle Naturschutzpraxis, insbesondere für das FFH-Monitoring . -

Evolution of Deceit by Worthless Donations in a Nuptial Gift-Giving Spider
Current Zoology 60 (1): 43–51, 2014 Evolution of deceit by worthless donations in a nuptial gift-giving spider Paolo Giovanni GHISLANDI1, Maria J. ALBO1, 2, Cristina TUNI1, Trine BILDE1* 1 Department of Bioscience, Aarhus University, 8000, Aarhus C, Denmark 2 Laboratorio de Etología, Ecología y Evolución, IIBCE, Uruguay Abstract Males of the nursery web spider Pisaura mirabilis usually offer an insect prey wrapped in white silk as a nuptial gift to facilitate copulation. Males exploit female foraging preferences in a sexual context as females feed on the gift during copula- tion. It is possible for males to copulate without a gift, however strong female preference for the gift leads to dramatically higher mating success for gift-giving males. Females are polyandrous, and gift-giving males achieve higher mating success, longer copulations, and increased sperm transfer that confer advantages in sperm competition. Intriguingly, field studies show that ap- proximately one third of males carry a worthless gift consisting of dry and empty insect exoskeletons or plant fragments wrapped in white silk. Silk wrapping disguises gift content and females are able to disclose gift content only after accepting and feeding on the gift, meanwhile males succeed in transferring sperm. The evolution of deceit by worthless gift donation may be favoured by strong intra-sexual competition and costs of gift-construction including prey capture, lost foraging opportunities and investment in silk wrapping. Females that receive empty worthless gifts terminate copulation sooner, which reduces sperm transfer and likely disadvantages males in sperm competition. The gift-giving trait may thus become a target of sexually antagonistic co-evolution, where deceit by worthless gifts leads to female resistance to the trait. -

Crossness Sewage Treatment Works Nature Reserve & Southern Marsh Aquatic Invertebrate Survey
Commissioned by Thames Water Utilities Limited Clearwater Court Vastern Road Reading RG1 8DB CROSSNESS SEWAGE TREATMENT WORKS NATURE RESERVE & SOUTHERN MARSH AQUATIC INVERTEBRATE SURVEY Report number: CPA18054 JULY 2019 Prepared by Colin Plant Associates (UK) Consultant Entomologists 30a Alexandra Rd London N8 0PP 1 1 INTRODUCTION, BACKGROUND AND METHODOLOGY 1.1 Introduction and background 1.1.1 On 30th May 2018 Colin Plant Associates (UK) were commissioned by Biodiversity Team Manager, Karen Sutton on behalf of Thames Water Utilities Ltd. to undertake aquatic invertebrate sampling at Crossness Sewage Treatment Works on Erith Marshes, Kent. This survey was to mirror the locations and methodology of a previous survey undertaken during autumn 2016 and spring 2017. Colin Plant Associates also undertook the aquatic invertebrate sampling of this previous survey. 1.1.2 The 2016-17 aquatic survey was commissioned with the primary objective of establishing a baseline aquatic invertebrate species inventory and to determine the quality of the aquatic habitats present across both the Nature Reserve and Southern Marsh areas of the Crossness Sewage Treatment Works. The surveyors were asked to sample at twenty-four, pre-selected sample station locations, twelve in each area. Aquatic Coleoptera and Heteroptera (beetles and true bugs) were selected as target groups. A report of the previous survey was submitted in Sept 2017 (Plant 2017). 1.1.3 During December 2017 a large-scale pollution event took place and untreated sewage escaped into a section of the Crossness Nature Reserve. The primary point of egress was Nature Reserve Sample Station 1 (NR1) though because of the connectivity of much of the waterbody network on the marsh other areas were affected. -

Metacommunities and Biodiversity Patterns in Mediterranean Temporary Ponds: the Role of Pond Size, Network Connectivity and Dispersal Mode
METACOMMUNITIES AND BIODIVERSITY PATTERNS IN MEDITERRANEAN TEMPORARY PONDS: THE ROLE OF POND SIZE, NETWORK CONNECTIVITY AND DISPERSAL MODE Irene Tornero Pinilla Per citar o enllaçar aquest document: Para citar o enlazar este documento: Use this url to cite or link to this publication: http://www.tdx.cat/handle/10803/670096 http://creativecommons.org/licenses/by-nc/4.0/deed.ca Aquesta obra està subjecta a una llicència Creative Commons Reconeixement- NoComercial Esta obra está bajo una licencia Creative Commons Reconocimiento-NoComercial This work is licensed under a Creative Commons Attribution-NonCommercial licence DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode Irene Tornero Pinilla 2020 DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode IRENE TORNERO PINILLA 2020 DOCTORAL PROGRAMME IN WATER SCIENCE AND TECHNOLOGY SUPERVISED BY DR DANI BOIX MASAFRET DR STÉPHANIE GASCÓN GARCIA Thesis submitted in fulfilment of the requirements to obtain the Degree of Doctor at the University of Girona Dr Dani Boix Masafret and Dr Stéphanie Gascón Garcia, from the University of Girona, DECLARE: That the thesis entitled Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode submitted by Irene Tornero Pinilla to obtain a doctoral degree has been completed under our supervision. In witness thereof, we hereby sign this document. Dr Dani Boix Masafret Dr Stéphanie Gascón Garcia Girona, 22nd November 2019 A mi familia Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. -

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -

Bijdragen Tot De Dierkunde, 46 (2) - 1977
Site selection and growth of the larvae ofEylais discreta Koenike, 1897 (Acari, Hydrachnellae) by C. Davids G.J. Nielsen & P. Gehring Zoological Laboratory, University ofAmsterdam, The Netherlands Abstract Such differences in growth rate are known for Arrenurus papillator (O. F. Müller, 1776). The The distribution of the larvae of Eylais discreta on the larvae of this species have a larger size on the abdominal tergites of the host species Sigara striata, S. falleni subcosta than the anal veins of of on the wings and Cymatia coleoptrata is examined. dragonflies (Münchberg, 1963). The same holds On S. striata and S. falleni the segments 3 and 4, on C. true larvae of are for the Limnochares aquatica coleoptrata the segments 2 and 3 successively preferred. There is but little influence of multiple infestations on this (Linnaeus, 1758) attached on the prothorax of distribution. with those Gerridae, compared on the meso- and The growth rate on the various tergites of S. striata is metathorax (Pahnke, 1974). similar, while on C. coleoptrata a possible difference could not When studying these phenomena, one has to be proved statistically. The larvae of E. discreta generally study the first. hibernate on the host; during this phase there is little growth; growth not until March-April they start growing faster. The larvae on C. coleoptrataare retarded in growth compared to those on S. METHODS AND RESULTS striata. The show in larvae on S. falleni do not any increase size; this water is immune to infestation various bug species by Host insects were collected by dip net on several Hydrachnellae. -
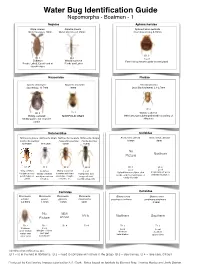
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Buglife Ditches Report Vol1
The ecological status of ditch systems An investigation into the current status of the aquatic invertebrate and plant communities of grazing marsh ditch systems in England and Wales Technical Report Volume 1 Summary of methods and major findings C.M. Drake N.F Stewart M.A. Palmer V.L. Kindemba September 2010 Buglife – The Invertebrate Conservation Trust 1 Little whirlpool ram’s-horn snail ( Anisus vorticulus ) © Roger Key This report should be cited as: Drake, C.M, Stewart, N.F., Palmer, M.A. & Kindemba, V. L. (2010) The ecological status of ditch systems: an investigation into the current status of the aquatic invertebrate and plant communities of grazing marsh ditch systems in England and Wales. Technical Report. Buglife – The Invertebrate Conservation Trust, Peterborough. ISBN: 1-904878-98-8 2 Contents Volume 1 Acknowledgements 5 Executive summary 6 1 Introduction 8 1.1 The national context 8 1.2 Previous relevant studies 8 1.3 The core project 9 1.4 Companion projects 10 2 Overview of methods 12 2.1 Site selection 12 2.2 Survey coverage 14 2.3 Field survey methods 17 2.4 Data storage 17 2.5 Classification and evaluation techniques 19 2.6 Repeat sampling of ditches in Somerset 19 2.7 Investigation of change over time 20 3 Botanical classification of ditches 21 3.1 Methods 21 3.2 Results 22 3.3 Explanatory environmental variables and vegetation characteristics 26 3.4 Comparison with previous ditch vegetation classifications 30 3.5 Affinities with the National Vegetation Classification 32 Botanical classification of ditches: key points -

Scottish Spiders - Oonopspulcher 15Mm
Scottish Spiders BeesIntroduction and wasps to spider families There are approximately 670 species of spider in 38 different families in the UK. This guide introduces 17 families of spiders, providing an example of a species or genus to look for in each. Please Note: The vast majority of spiders in the UK need examination under a microscope of mature adults to confirm species. Immature specimens may be identified to family or to genus level and often only by an expert. This guide has been designed to introduce several families with information on key features in each and is not an identification guide. Woodlouse spiders (Family Dysderidae) 4 species in 2 genera Rather elongate looking spiders with no clear markings or Woodlouse spider (female) pattern on their cylindrical abdomen. They have six eyes that are clustered together in a circular formation. Often found under stones, logs, tree bark and other debris. Typical body length in family ranges from 6-15mm. Species to look out for - Woodlouse spider (Dysdera crocata) A distinctive species with a red cephalothorax and legs and forward projecting chelicerae. This species feeds on woodlice and can be found under stones and debris in warm (and sometimes) slightly damp situations. Generally nocturnal - look for them in gardens and on walls where they may be found sheltering in silken retreats. This species is common in England but less so in Scotland, being absent from the very north. Look out for Harpactea hombergi which although similar in Male: 9—10mm Female: 11—15mm appearance has a narrower cephalothorax and with less Falk © Steven prominent chelicerae. -

Variations on a Theme
HENRY JOUTSIJOKI Variations on a Theme The Classification of Benthic Macroinvertebrates ACADEMIC DISSERTATION To be presented, with the permission of the board of the School of Information Sciences of the University of Tampere, for public discussion in the Auditorium Pinni B 1100, Kanslerinrinne 1, Tampere, on November 9th, 2012, at 12 o’clock. UNIVERSITY OF TAMPERE ACADEMIC DISSERTATION University of Tampere School of Information Sciences Finland Copyright ©2012 Tampere University Press and the author Distribution Tel. +358 40 190 9800 Bookshop TAJU [email protected] P.O. Box 617 www.uta.fi/taju 33014 University of Tampere http://granum.uta.fi Finland Cover design by Mikko Reinikka Acta Universitatis Tamperensis 1777 Acta Electronica Universitatis Tamperensis 1251 ISBN 978-951-44-8952-5 (print) ISBN 978-951-44-8953-2 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://acta.uta.fi Tampereen Yliopistopaino Oy – Juvenes Print Tampere 2012 Abstract This thesis focused on the classification of benthic macroinvertebrates by us- ing machine learning methods. Special emphasis was placed on multi-class extensions of Support Vector Machines (SVMs). Benthic macroinvertebrates are used in biomonitoring due to their properties to react to changes in water quality. The use of benthic macroinvertebrates in biomonitoring requires a large number of collected samples. Traditionally benthic macroinvertebrates are separated and identified manually one by one from samples collected by biologists. This, however, is a time-consuming and expensive approach. By the automation of the identification process time and money would be saved and more extensive biomonitoring would be possible. The aim of the thesis was to examine what classification method would be the most appro- priate for automated benthic macroinvertebrate classification.