Of the Retina with Tearing of the Macula Lutea* by M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -
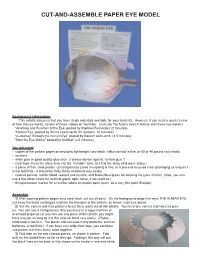
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

Scleral Lenses and Eye Health
Scleral Lenses and Eye Health Anatomy and Function of the Human Eye How Scleral Lenses Interact with the Ocular Surface Just as the skin protects the human body, the ocular surface protects the human Scleral lenses are large-diameter lenses designed to vault the cornea and rest on the conjunctival tissue sitting on eye. The ocular surface is made up of the cornea, the conjunctiva, the tear film, top of the sclera. The space between the back surface of the lens and the cornea acts as a fluid reservoir. Scleral and the glands that produce tears, oils, and mucus in the tear film. lenses can range in size from 13mm to 19mm, although larger diameter lenses may be designed for patients with more severe eye conditions. Due to their size, scleral lenses consist SCLERA: The sclera is the white outer wall of the eye. It is SCLERAL LENS made of collagen fibers that are arranged for strength rather of at least two zones: than transmission of light. OPTIC ZONE The optic zone vaults over the cornea CORNEA: The cornea is the front center portion of the outer Cross section of FLUID RESERVOIR wall of the eye. It is made of collagen fibers that are arranged in the eye shows The haptic zone rests on the conjunctiva such a way so that the cornea is clear. The cornea bends light the cornea, overlying the sclera as it enters the eye so that the light is focused on the retina. conjunctiva, and sclera as CORNEA The cornea has a protective surface layer called the epithelium. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Corneal Erosion?
What Is the Cornea? The cornea is the clear front window of the eye. It covers the iris (colored portion of the eye) and the round pupil, much like a watch crystal covers the face of a watch. The cornea is composed of five layers. The outermost surface layer is called the epithelium. Normal Eye Anatomy What Is a Corneal Abrasion? A corneal abrasion is an injury (a scratch, scrape or cut) to the corneal epithelium. Abrasions are commonly caused by fingernail scratches, paper cuts, makeup brushes, scrapes from tree or bush limbs, and rubbing of the eye. Some eye conditions, such as dry eye, increase the chance of an abrasion. You may experience the following symptoms with corneal abrasion: • Feeling of having something in your eye • Pain and soreness of the eye • Redness of the eye • Sensitivity to light • Tearing • Blurred vision To detect an abrasion on the cornea, your ophthalmologist (Eye M.D.) will use a special dye called fluorescein (pronounced FLOR-uh-seen) to illuminate the injury. How Is a Corneal Abrasion Treated? Treatment may include the following: • Patching the injured eye to prevent eyelid blinking from irritating the injury. • Applying lubricating eyedrops or ointment to the eye to form a soothing layer between the eyelid and the abrasion. • Using antibiotics to prevent infection. • Dilating (widening) the pupil to relieve pain. • Wearing a special contact lens to help healing. Minor abrasions usually heal within a day or two; larger abrasions usually take about a week. It is important not to rub the eye while it is healing. -

The Role of the Sclera and Orbital Tissues in the Biomechanical Deformation Response of the Cornea and Whole Eye Under Loading B
The Role of the Sclera and Orbital Tissues in the Biomechanical Deformation Response of the Cornea and Whole Eye Under Loading by Dynamic Scheimpflug Analyzer Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Boihoan Audrey Nguyen, M.S. Graduate Program in Biomedical Engineering The Ohio State University 2019 Dissertation Committee Cynthia J. Roberts, Ph.D, Advisor Matthew A. Reilly, Ph.D., Co-advisor Jun Liu, Ph.D., Committee Member Copyrighted by Boihoan Audrey Nguyen 2019 2 Abstract The biomechanical behavior of the ocular and orbital tissues are important to maintaining proper structure and function of the eye. The cornea and the sclera are the two main components of the ocular shell, which are loaded by IOP and are responsible for maintaining the structure and therefore function of the eye. This work comprises of four studies which explore the contribution of the sclera and the biomechanical response of the eye. The first study focused on the development of a finite-element model to explore the impact of varying scleral properties on the deformation response of the cornea to an air- puff. An axisymmetric model of the eye – consisting of a cornea, sclera, and vitreous humor – loaded internally by intraocular pressure (IOP) and externally by an air-puff from a noncontact tonometer was generated in COMSOL 5.2a. Our results showed that increasing scleral stiffness (with constant corneal stiffness and IOP) resulted in decreasing displacement of the corneal apex, i.e. the cornea responded to the air-puff as if it had stiffer mechanical properties. -

MACULAR HOLE What Is the Macular? the Back of the Eye Has a Light-Sensitive Lining Called the Retina, Similar to the Film in a Camera
MACULAR HOLE What is the macular? The back of the eye has a light-sensitive lining called the retina, similar to the film in a camera. Light is focussed through the eye onto the retina, allowing us to see. The centre part of the retina is called the macula- it is here that light must be focused for us to see fine detail to be able to read and see in colour. Retina Cornea Macula Lens Optic Nerve Vitreous Body What is a macular hole? A macular hole is a small, circular gap which opens up at the centre of the retina. This causes blurred and often distorted vision where straight lines or letters look wavy or bowed. There may also be a patch of missing vision at the centre. Is a macular hole the same as age-related macular degeneration? No, they are different conditions although they affect the same area of the eye. They can sometimes both be present in the same eye. Normal Macular OCT scan Macular hole OCT scan Why does it happen? We don’t know why macular holes develop. They most often occur in people aged 60-80, and is twice as common in women as men. We are increasingly aware that it is mainly longsighted people who are affected. Other causes of macular holes include severe trauma to the eye, being very shortsighted (myopic), those who have had a retinal detachment or as a result of a longstanding swelling of the central retina (cystoid macular oedema). What would happen if I did not have my macular hole treated? If untreated, there is a small chance that some macular holes can close spontaneously, with improvement in vision. -
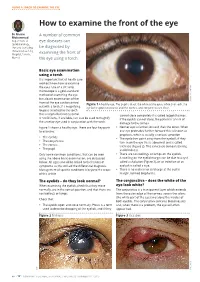
How to Examine the Front of the Eye
USING A TORCH TO EXAMINE THE EYE How to examine the front of the eye Dr Nasiru A number of common Muhammad Department of eye diseases can Ophthalmology, Usmanu Danfodiyo be diagnosed by University Teaching Hospital, Sokoto, examining the front of Nigeria. the eye using a torch. Basic eye examination using a torch It is important that all health care workers know how to examine the eyes. Use of a slit lamp microscope is a gold-standard method of examining the eye but a basic examination of the front of the eye can be carried SANDIP DAS SANYAM Figure 1 A healthy eye. The pupil is black, the white of the eye is white (not red), the out with a torch; if a magnifying eyelashes point outwards and the cornea and conjunctiva are clear. loupe is attached to the torch this is helpful but not essential. cannot close completely it is called lagophthalmos. A +20 DS lens, if available, can also be used to magnify If the eyelids cannot close, the patient is at risk of the anterior eye used in conjunction with the torch. damage to the cornea. Figure 1 shows a healthy eye. There are four key parts • Neither eye is further forward than the other. When to examine: one eye protrudes further forward this is known as proptosis, which is usually a serious condition. • The eyelids • The eyelashes point away from the eyeball; if they • The conjunctiva turn in on the eye this is abnormal and is called • The cornea trichiasis (Figure 2). This can cause corneal scarring • The pupil and blindness. -

Corneal Sensitivity and Substance P in Experimental Herpes Simplex Keratitis in Mice
Corneal Sensitivity and Substance P in Experimental Herpes Simplex Keratitis in Mice Andrew B. Tullo,* Perer Keeaf William A. Dlyrh.J Terry J. H1II4 and David L. Easry* Experimental herpes simplex keratitis in the mouse produced a rapid fall in both corneal sensitivity and levels of corneal substance P (SP). This finding supports the association of SP with sensory neurones and shows that such levels can be used as an indication of damage to neurones resulting, for example, from infection with HSV. However, the delay in recovery of SP compared to the more rapid and complete recovery of sensitivity suggests that SP in the cornea is not directly involved in mediation of the blink reflex. Invest Ophthalmol Vis Sci 24:596-598, 1983 Neuropeptides are currently receiving much atten- 4 weeks previously,8 were inoculated by corneal scar- tion because of their widespread distribution within ification of the left eye7 through 5 n\ of growth me- the nervous system and because of their possible role dium containing 3.5 X 106 pfu of the same strain of in neurotransmission. One of these small peptides virus. As controls the corneas of uninfected mice were substance P (SP) is known to be particularly strongly scarified through medium without virus. Scarification associated with primary afferent neurones.1 It is to of the cornea in both groups consisted often parallel be found not only at the central terminals of such strokes with a 26-gauge needle followed by a further nerve fibers, but also in the peripheral endings,2 and ten perpendicular to the first. -

Corneal Anatomy
FFCCF! • Mantis Shrimp have 16 cone types- we humans have three- essentially Red Green and Blue receptors. Whereas a dog has 2, a butterfly has 5, the Mantis Shrimp may well see the most color of any animal on earth. Functional Morphology of the Vertebrate Eye Christopher J Murphy DVM, PhD, DACVO Schools of Medicine & Veterinary Medicine University of California, Davis With integrated FFCCFs Why Does Knowing the Functional Morphology Matter? • The diagnosis of ocular disease relies predominantly on physical findings by the clinician (maybe more than any other specialty) • The tools we routinely employ to examine the eye of patients provide us with the ability to resolve fine anatomic detail • Advanced imaging tools such as optical coherence tomography (OCT) provide very fine resolution of structures in the living patient using non invasive techniques and are becoming widespread in application http://dogtime.com/trending/17524-organization-to-provide-free-eye-exams-to-service- • The basis of any diagnosis of “abnormal” is animals-in-may rooted in absolute confidence of owning the knowledge of “normal”. • If you don’t “own” the knowledge of the terminology and normal functional morphology of the eye you will not be able to adequately describe your findings Why Does Knowing the Functional Morphology Matter? • The diagnosis of ocular disease relies predominantly on physical findings by the clinician (maybe more than any other specialty) • The tools we routinely employ to examine the eye of patients provide us with the ability to resolve fine anatomic detail http://www.vet.upenn.edu/about/press-room/press-releases/article/ • Advanced imaging tools such as optical penn-vet-ophthalmologists-offer-free-eye-exams-for-service-dogs coherence tomography (OCT) provide very fine resolution of structures in the living patient using non invasive techniques and are becoming widespread in application • The basis of any diagnosis of “abnormal” is rooted in absolute confidence of owning the http://aibolita.com/eye-diseases/37593-direct-ophthalmoscopy.html knowledge of “normal”. -

A Single Cell Atlas of Human Cornea That Defines Its Development, Limbal Stem and Progenitor Cells and the Interactions with the Limbal Niche
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.09.195438; this version posted July 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A single cell atlas of human cornea that defines its development, limbal stem and progenitor cells and the interactions with the limbal niche Joseph Collin1+, Rachel Queen 1+, Darin Zerti 1,2+, Sanja Bojic 1, Nicky Moyse 3, Marina Moya Molina 1, Chunbo Yang1, Gary Reynolds 1, Rafiqul Hussain 1, Jonathan M Coxhead 1, Steven Lisgo 1, Deborah Henderson 1, Agatha Joseph 4, Paul Rooney 4, Saurabh Ghosh 5, Che Connon 1, Muzlifah Haniffa 1, Francisco Figueiredo 6, Lyle Armstrong 1 and Majlinda Lako1* 1. Biosciences Institute, Faculty of Medical Sciences, Newcastle University, UK 2. Present address: Microscopy Centre and Department of Applied Clinical Sciences and Biotechnology, University of L’Aquila, Italy 3. Newcastle Cellular Therapies Facility, Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust, UK 4. NHS Blood and Transplant Tissue and Eye Services, Liverpool, UK 5. Royal Victoria Infirmary, Sunderland, UK 6. Department of Ophthalmology, Royal Victoria Infirmary and Newcastle University, Newcastle, UK + these authors contributed equally *author for correspondence ([email protected]) Keywords: embryonic and fetal eye, cornea, conjunctiva, ocular surface, single cell RNA- Seq, single cell ATAC-Seq, LSC dysplasia, keratoconus, limbal epithelial expansion, limbal stem cells, transit amplifying cells. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.09.195438; this version posted July 10, 2020.