Cell–Matrix Interactions in the Eye: from Cornea to Choroid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

12 Retina Gabriele K
299 12 Retina Gabriele K. Lang and Gerhard K. Lang 12.1 Basic Knowledge The retina is the innermost of three successive layers of the globe. It comprises two parts: ❖ A photoreceptive part (pars optica retinae), comprising the first nine of the 10 layers listed below. ❖ A nonreceptive part (pars caeca retinae) forming the epithelium of the cil- iary body and iris. The pars optica retinae merges with the pars ceca retinae at the ora serrata. Embryology: The retina develops from a diverticulum of the forebrain (proen- cephalon). Optic vesicles develop which then invaginate to form a double- walled bowl, the optic cup. The outer wall becomes the pigment epithelium, and the inner wall later differentiates into the nine layers of the retina. The retina remains linked to the forebrain throughout life through a structure known as the retinohypothalamic tract. Thickness of the retina (Fig. 12.1) Layers of the retina: Moving inward along the path of incident light, the individual layers of the retina are as follows (Fig. 12.2): 1. Inner limiting membrane (glial cell fibers separating the retina from the vitreous body). 2. Layer of optic nerve fibers (axons of the third neuron). 3. Layer of ganglion cells (cell nuclei of the multipolar ganglion cells of the third neuron; “data acquisition system”). 4. Inner plexiform layer (synapses between the axons of the second neuron and dendrites of the third neuron). 5. Inner nuclear layer (cell nuclei of the bipolar nerve cells of the second neuron, horizontal cells, and amacrine cells). 6. Outer plexiform layer (synapses between the axons of the first neuron and dendrites of the second neuron). -

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

Current Trends in Ophthalmology Autonomic Regulation of The
Review Article Current Trends in Ophthalmology Autonomic Regulation of the Function of the Vitreous Body and Retina Lychkova AE1*, Severin AE2, Torshin VI2, Starshinov YP2, Sdobnikova SV3, Ashrafov RA4, Ashrafova SR4, Golubev YY5 Golubeva GY6 and Puzikov AM1 1Department of Health, Moscow’s Clinical Research Center, Moscow, Russia 2Russian People’s Friendship University, Moscow, Russia 3Research Institute of Eye Diseases, Moscow, Russia 4Center for Laser Surgery, Moscow, Russia 5Russian National Research Medical University, Moscow, Russia 6City Clinical Hospital, Moscow, Russia *Correspondence: Lychkova Alla Edward, Department Head of the Moscow’s Clinical Research Center, DZM, Shosse Enthusiasts 86, 111123, 11-1-53, Amundsen 129343, Moscow, Russia, Tel: (+7) 962-965-4923; E-mail: [email protected] Received: May 08, 2018; Accepted: June 25, 2018; Published: June 29, 2018 Abstract The data of the literature on the structure and physiology of the vitreous body and the retina in normal and pathological conditions are presented. The mechanism of vitreous detachment and its role in the development of vitreoretinal proliferation are described. Described adren-choline-peptide and NO-ergic mechanisms in signal transduction of the vitreous body and retina. Pharmacological and surgical methods of treatment of vitreoretinal proliferation are briefly described. Keywords: Vitreous body; Retina; Detachment; Vitreoretinal proliferation Introduction density of collagen fibers and a greater concentration of hyaluronic acid as compared to the central part. Cortical Vitreous Body (VB) is a transparent, colourless, gel- gel is comparatively more stable and more resistant to like mass containing water with an admixture of salts, age-related changes [1]. The VB contains two channels sugars, hyaluronic acid, a network of collagen fibers II, IX, (optociliary and lenticular) and three rows of cisterns, a XI types and few cells (mainly phagocytes, contributing to bursa premacularis and a precapillary space [1,3-5]. -

Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
pharmaceutics Article Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors Hyeong Min Kim 1,†, Hyounkoo Han 2,†, Hye Kyoung Hong 1, Ji Hyun Park 1, Kyu Hyung Park 1, Hyuncheol Kim 2,* and Se Joon Woo 1,* 1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 13620, Korea; [email protected] (H.M.K.); [email protected] (H.K.H.); [email protected] (J.H.P.); [email protected] (K.H.P.) 2 Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Korea; [email protected] * Correspondence: [email protected] (H.K.); [email protected] (S.J.W.); Tel.: +82-2-705-8922 (H.K.); +82-31-787-7377 (S.J.W.); Fax: +82-2-3273-0331 (H.K.); +82-31-787-4057 (S.J.W.) † These authors contributed equally to this work. Abstract: In this study, Retina-RPE-Choroid-Sclera (RCS) and RPE-Choroid-Sclera (CS) were prepared by scraping them off neural retina, and using the Ussing chamber we measured the average time– concentration values in the acceptor chamber across five isolated rabbit tissues for each drug molecule. We determined the outward direction permeability of the RCS and CS and calculated the neural retina permeability. The permeability coefficients of RCS and CS were as follows: ganciclovir, 13.78 ± 5.82 and 23.22 ± 9.74; brimonidine, 15.34 ± 7.64 and 31.56 ± 12.46; bevacizumab, 0.0136 ± 0.0059 and 0.0612 ± 0.0264 (×10−6 cm/s). -

Endothelium Ii
Br J Ophthalmol: first published as 10.1136/bjo.42.11.667 on 1 November 1958. Downloaded from Brit. J. Ophthal. (1958) 42, 667. STUDIES ON THE CORNEAL AND TRABECULAR ENDOTHELIUM II. ENDOTHELIUM OF THE ZONE OF TRANSITION* BY F. VRABEC From the First Eye Clinic, University ofPrague, Czechoslovakia AT the periphery of the cornea the corneal endothelium passes over the margin of Descemet's membrane to the trabecular meshwork (Vrabec, 1957). The size and shape of the cells of the corneal endothelium undergo a peculiar change in approaching this region (Vrabec, 1958a). A study by means of the replica method (Vrabec, 1958b) demonstrated that the endothelial cells became elongated in the meridional direction and then lost their outlines in the region of the anterior border of Schwalbe's ring. Only a few nuclei were seen by the replica technique. The results of examining the endothelium of this zone of transition by various methods is described below. copyright. Material and Methods The eyes of the cat, rabbit, and rhesus monkey were studied, together with some human eyes with different pathological conditions, and one human eye which was clinically niormal but was enucleated because of an orbital tumour. The basic method used was again the silver impregnation method of McGovern (1955, 1956). The results were compared with those obtained by the replica and pseudo-replica methods, the latter mostly with additional staining. Even in the http://bjo.bmj.com/ apparently normal eye, the possibility of functional changes in the secretion of the cement substance as well as of the covering substance should be borne in mind. -

Corneal Endothelium Endothelium Cells Are Destroyed by Disease Or Trauma, Thelial Cells Per Year
Integrating the Best Davis EyeCare Technology Associates Specular Microscopy is a new tech- nique to monitor corneal cell loss due to damage from extended con- tact lens wear, surgery, or the ag- ing process. It is also an excellent tool for: educating patients, screening for corneal disease (fuchs-guttata, kerataconus, Corneal trauma, dry eye, glaucoma, diabe- tes and certain medications etc.) and observing the damaging ef- Endothelium fects of contact lens wear. Often we can avoid more serious complica- tions of con- tact lens wear by under- standing the condition of the cornea. In early stages simply ad- justing the wearing time or chang- ing to a different contact lens ma- terial avoids future issues. In our elderly population our cell counts diminish and with a specular mi- croscope we can monitor and treat Davis EyeCare Associates the aging cornea much more effec- tively. This is an essential tool in managing our contact lens patients 4663 West 95th Street www.daviseyecare.com and is very important in assessing Oak Lawn Il 60453 potential risks for cataracts and Phone: 708-636-0600 4663 West 95th Street refractive surgery. Oak Lawn IL 60453 Fax: 708-636-0606 708-636-0600 E-mail: www.daviseyecare.com mal aging, the central cornea loses 100 to 500 endo- Corneal Endothelium endothelium cells are destroyed by disease or trauma, thelial cells per year. When these cells die, they they are lost forever. slough off the posterior surface of the cornea into the We are pleased to offer Konan Microscopy to the anterior chamber, creating a gap in the endothelial Common ocular conditions, such as glaucoma, uveitis management of your eyecare. -

Tissue Engineering of the Corneal Endothelium: a Review of Carrier Materials
J. Funct. Biomater. 2013, 4, 178-208; doi:10.3390/jfb4040178 Journal of Functional Biomaterials ISSN 2079-4983 www.mdpi.com/journal/jfb/ Review Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials Juliane Teichmann 1,2,†, Monika Valtink 2,†,*, Mirko Nitschke 1, Stefan Gramm 1, Richard H.W. Funk 2,3, Katrin Engelmann 3,4 and Carsten Werner 1,3 1 Leibniz Institute of Polymer Research Dresden, Max Bergmann Center of Biomaterials, Institute of Biofunctional Polymer Materials, Hohe Straße 6, Dresden 01069, Germany; E-Mails: [email protected] (J.T.); [email protected] (M.N.); [email protected] (S.G.); [email protected] (C.W.) 2 Institute of Anatomy, Medical Faculty Carl Gustav Carus, Technische Universität Dresden, Fetscherstraße 74, Dresden 01307, Germany; E-Mail: [email protected] 3 CRTD/DFG-Center for Regenerative Therapies Dresden—Cluster of Excellence, Fetscherstraße 105, Dresden 01307, Germany 4 Department of Ophthalmology, Klinikum Chemnitz gGmbH, Flemmingstraße 2, Chemnitz 09116, Germany; E-Mail: [email protected] † These authors contribute equally to the work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-351-458-6124; Fax: +49-351-458-6303. Received: 3 August 2013; in revised form: 13 September 2013 / Accepted: 24 September 2013 / Published: 22 October 2013 Abstract: Functional impairment of the human corneal endothelium can lead to corneal blindness. In order to meet the high demand for transplants with an appropriate human corneal endothelial cell density as a prerequisite for corneal function, several tissue engineering techniques have been developed to generate transplantable endothelial cell sheets. -

The Distribution of Immune Cells in the Uveal Tract of the Normal Eye
THE DISTRIBUTION OF IMMUNE CELLS IN THE UVEAL TRACT OF THE NORMAL EYE PAUL G. McMENAMIN Perth, Western Australia SUMMARY function of these cells in the normal iris, ciliary body Inflammatory and immune-mediated diseases of the and choroid. The role of such cell types in ocular eye are not purely the consequence of infiltrating inflammation, which will be discussed by other inflammatory cells but may be initiated or propagated authors in this issue, is not the major focus of this by immune cells which are resident or trafficking review; however, a few issues will be briefly through the normal eye. The uveal tract in particular considered where appropriate. is the major site of many such cells, including resident tissue macro phages, dendritic cells and mast cells. This MACRO PHAGES review considers the distribution and location of these and other cells in the iris, ciliary body and choroid in Mononuclear phagocytes arise from bone marrow the normal eye. The uveal tract contains rich networks precursors and after a brief journey in the blood as of both resident macrophages and MHe class 11+ monocytes immigrate into tissues to become macro dendritic cells. The latter appear strategically located to phages. In their mature form they are widely act as sentinels for capturing and sampling blood-borne distributed throughout the body. Macrophages are and intraocular antigens. Large numbers of mast cells professional phagocytes and play a pivotal role as are present in the choroid of most species but are effector cells in cell-mediated immunity and inflam virtually absent from the anterior uvea in many mation.1 In addition, due to their active secretion of a laboratory animals; however, the human iris does range of important biologically active molecules such contain mast cells. -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -

Management of Post Operative Seclusio Pupil and Corneal Decompensation
MedDocs Publishers Annals of Ophthalmology and Visual Sciences Open Access | Case Report Management of post operative seclusio pupil and corneal decompensation Arjun Srirampur, MS, FRCS*; Kavya Reddy, MS; Aruna Kumari Gadde, MS; Sunny Manwani, DNB Anand Eye Institue, Habsiguda, Hyderabad, India *Corresponding Author(s): Arjun Srirampur, Abstract Anand Eye Institue, Habsiguda, Hyderabad, India A 71 year old man presented with a history of cataract Email: [email protected] surgery in both eyes and gradual diminution of vision post surgery in right eye (RE) since 3 years. On examination RE showed corneal decompensation with seclusio pupillae Received: Jan 25, 2018 with a small pupil and a posterior chamber intraocular lens (PCIOL). He was diagnosed with pseudophakic bullous ker- Accepted: Apr 20, 2018 atopathy (PBK). He underwent DSAEK (Descemet’s stripping Published Online: Apr 27, 2018 automated endothelial keratoplasty ) with synechiolysis and Journal: Annals of Ophthalmology and Visual Sciences pupilloplasty. Graft lenticule was well attached to the host Publisher: MedDocs Publishers LLC tissue with a vertically oval pupil and subsequent improve- ment of vision. Online edition: http://meddocsonline.org/ Copyright: © Srirampur A (2018). This Article is distributed under the terms of Creative Commons Attribution 4.0 international License Keywords: Seclusio pupil; Corneal decompensation ; De- scemet’s stripping automated endothelial keratoplasty Introduction More recently, long-term follow-up has revealed the exis- tence of progressive changes in corneal endothelium following PBK may occur in around 1 to 2% of the patients undergo- intraocular lens insertion. Though the pathogenesis of this phe- ing cataract surgery, which accounts two to four million patients nomenon is not clear, persistent low grade inflammation and worldwide. -

Vitreous and Developmental Vitreoretinopathies Kevin R
CHAPTER 3 Vitreous and Developmental Vitreoretinopathies Kevin R. Tozer, Kenneth M. P. Yee, and J. Sebag Invisible (Fig. 3.1) by design, vitreous was long unseen the central vitreous and adjacent to the anterior cortical as important in the physiology and pathology of the eye. gel. HA molecules have a different distribution from col- Recent studies have determined that vitreous plays a sig- lagen, being most abundant in the posterior cortical gel nificant role in ocular health (1) and disease (1,2), includ- with a gradient of decreasing concentration centrally ing a number of important vitreoretinal disorders that and anteriorly (6,7). arise from abnormal embryogenesis and development. Both collagen and HA are synthesized during child- Vitreous embryology is presented in detail in Chapter 1. hood. Total collagen content in the vitreous gel remains Notable is that primary vitreous is filled with blood ves- at about 0.05 mg until the third decade (8). As collagen sels during the first trimester (Fig. 3.2). During the second does not appreciably increase during this time but the trimester, these vessels begin to disappear as the second- size of the vitreous does increase with growth, the den- ary vitreous is formed, ultimately resulting in an exqui- sity of collagen fibrils effectively decreases. This could sitely clear gel (Fig. 3.1). The following will review vitreous potentially weaken the collagen network and destabilize development and the congenital disorders that arise from the gel. However, since there is active synthesis of HA abnormalities in hyaloid vessel formation and regression during this time, the dramatic increase in HA concentra- during the primary vitreous stage and biochemical abnor- tion may stabilize the thinning collagen network (9). -
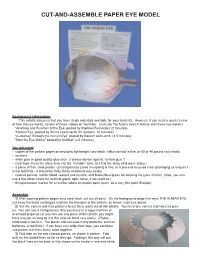
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above.