Visual System EYEBALL — Composed of Three Concentric Layers: 1] SCLERA (White) and CORNEA (Transparent) = Outer, Fibrous Layer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
pharmaceutics Article Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors Hyeong Min Kim 1,†, Hyounkoo Han 2,†, Hye Kyoung Hong 1, Ji Hyun Park 1, Kyu Hyung Park 1, Hyuncheol Kim 2,* and Se Joon Woo 1,* 1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 13620, Korea; [email protected] (H.M.K.); [email protected] (H.K.H.); [email protected] (J.H.P.); [email protected] (K.H.P.) 2 Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Korea; [email protected] * Correspondence: [email protected] (H.K.); [email protected] (S.J.W.); Tel.: +82-2-705-8922 (H.K.); +82-31-787-7377 (S.J.W.); Fax: +82-2-3273-0331 (H.K.); +82-31-787-4057 (S.J.W.) † These authors contributed equally to this work. Abstract: In this study, Retina-RPE-Choroid-Sclera (RCS) and RPE-Choroid-Sclera (CS) were prepared by scraping them off neural retina, and using the Ussing chamber we measured the average time– concentration values in the acceptor chamber across five isolated rabbit tissues for each drug molecule. We determined the outward direction permeability of the RCS and CS and calculated the neural retina permeability. The permeability coefficients of RCS and CS were as follows: ganciclovir, 13.78 ± 5.82 and 23.22 ± 9.74; brimonidine, 15.34 ± 7.64 and 31.56 ± 12.46; bevacizumab, 0.0136 ± 0.0059 and 0.0612 ± 0.0264 (×10−6 cm/s). -

Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts After Long-Duration Space Flight
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 10-2011 Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight Thomas H. Mader Alaska Native Medical Center, [email protected] C. Robert Gibson Coastal Eye Associates Anastas F. Pass University of Houston Larry A. Kramer University of Texas Health Science Center Andrew G. Lee The Methodist Hospital See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/nasapub Part of the Physical Sciences and Mathematics Commons Mader, Thomas H.; Gibson, C. Robert; Pass, Anastas F.; Kramer, Larry A.; Lee, Andrew G.; Fogarty, Jennifer; Tarver, William J.; Dervay, Joseph P.; Hamilton, Douglas R.; Sargsyan, Ashot; Phillips, John L.; Tran, Duc; Lipsky, William; Choi, Jung; Stern, Claudia; Kuyumjian, Raffi; andolk, P James D., "Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight" (2011). NASA Publications. 69. https://digitalcommons.unl.edu/nasapub/69 This Article is brought to you for free and open access by the National Aeronautics and Space Administration at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in NASA Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Thomas H. Mader, C. Robert Gibson, Anastas F. Pass, Larry A. -

The Sclera C
The Sclera c. Stephen Foster Maite Sainz de la Maza The Sclera Foreword by Frederick A. lakobiec With 134 Illustrations and 33 Color Plates Springer Science+Business Media, LLC C. Stephen Foster, MD Associate Professor of Ophthalmology Harvard Medical School Director, Immunology and Uveitis Service Massachusetts Eye and Ear Infirmary Boston, MA 02114 USA Maite Sainz de la Maza, MD, PhD Assistant Professor of Ophthalmology Central University of Barcelona 08036 Barcelona Spain Cover illustration: The eye of a patient with rheumatoid arthritis who has developed pro gressively destructive necrotizing scleritis. Library of Congress Cataloging-in-Publication Data Foster, C. Stephen (Charles Stephen), 1942- The sclera/C. Stephen Foster and Maite Sainz de la Maza. p. cm. Includes bibliographical references and index. ISBN 978-1-4757-2345-8 ISBN 978-1-4757-2343-4 (eBook) DOI 10.1007/978-1-4757-2343-4 1. Sclera-Diseases. I. Maza, Maite Sainz de lao II. Title. [DNLM: 1. Scleritis. 2. Sclera. WW 230 F754s 1993] RE328.F67 1993 617.7' 19-dc20 DNLMIDLC for Library of Congress 93-10235 Printed on acid-free paper. © 1994 Springer Science+ Business Media New York Originally published by Springer-Verlag New York, Inc. in 1994 Softcover reprint of the hardcover 1st edition 1994 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher, Springer Science+Business Media, LLC. except for brief excerpts in connection with reviews or, scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. -

How Clean Is Your Capsule?
Eye (1989) 3, 678-684 How Clean is Your Capsule? W. T. GREEN and D. L. BOASE Portsmouth Summary Proliferation of residual lens epithelial cells is believed to be the major cause of pos terior capsule opacification following extracapsular cataract extraction. During sur gery these cells can be visualised with appropriate illumination facilitating their mechanical removal with the McIntyre cannula. When flat preparations of the anterior capsule are examined by light microscopy, the areas 'cleaned' of cells in this way appear transparent but scanning electron microscopy reveals tufts of remaining debris which may represent points of cellular attachment to the capsule. Control of lens epithelial cell proliferation is important for the future development of cataract surgery. The undoubted advantages of extracapsular and also on human cadaver eyes. A horizontal cataract extraction are offset in many patients capsulotomy in the upper part of the lens by posterior capsule opacification requiring allowed nucleus removal. Irrigation and caps ulotomy. Not only is this disappointing aspiration of the cortical lens material was for the patient, but the procedure carries a then carried out using a McIntyre cannula risk of serious complications. with Hartman's irrigation solution. During in The major cause of posterior capsule opac vitro surgery this was aided by first removing ification is proliferation of residual lens epi the entire cornea and iris to improve visual thelial cells. I If these cells could be removed at isation and explore different methods of the time of surgery we believe that the inci illumination. dence of posterior capsule opacification and The importance of illumination was first the need for subsequent capsulotomy would suspected when it was observed, during rou be reduced. -

Selective Attention Within the Foveola
ARTICLES Selective attention within the foveola Martina Poletti1 , Michele Rucci1,2 & Marisa Carrasco3,4 Efficient control of attentional resources and high-acuity vision are both fundamental for survival. Shifts in visual attention are known to covertly enhance processing at locations away from the center of gaze, where visual resolution is low. It is unknown, however, whether selective spatial attention operates where the observer is already looking—that is, within the high-acuity foveola, the small yet disproportionally important rod-free region of the retina. Using new methods for precisely controlling retinal stimulation, here we show that covert attention flexibly improves and speeds up both detection and discrimination at loci only a fraction of a degree apart within the foveola. These findings reveal a surprisingly precise control of attention and its involvement in fine spatial vision. They show that the commonly studied covert shifts of attention away from the fovea are the expression of a global mechanism that exerts its action across the entire visual field. Covert attention is essential for visual perception. Among its many previous studies. We then investigated the consequences of attention advantages, covert allocation of attentional resources increases con- for both detection (experiment 2) and discrimination (experiments trast sensitivity and spatial resolution, speeds information accrual and 3 and 4) tasks within the foveola. reaction times1–4, and alters the signal at the target location during saccade preparation5–7. Covert attention has been studied sometimes RESULTS in the parafovea (1°–5°) and mostly in the perifovea (5°–10°) and Experiment 1 consisted of a central spatial cueing task with para- periphery (>10° of eccentricity)—that is, far outside the foveola, the foveal stimuli (Fig. -

Understanding Sensory Processing: Looking at Children's Behavior Through the Lens of Sensory Processing
Understanding Sensory Processing: Looking at Children’s Behavior Through the Lens of Sensory Processing Communities of Practice in Autism September 24, 2009 Charlottesville, VA Dianne Koontz Lowman, Ed.D. Early Childhood Coordinator Region 5 T/TAC James Madison University MSC 9002 Harrisonburg, VA 22807 [email protected] ______________________________________________________________________________ Dianne Koontz Lowman/[email protected]/2008 Page 1 Looking at Children’s Behavior Through the Lens of Sensory Processing Do you know a child like this? Travis is constantly moving, pushing, or chewing on things. The collar of his shirt and coat are always wet from chewing. When talking to people, he tends to push up against you. Or do you know another child? Sierra does not like to be hugged or kissed by anyone. She gets upset with other children bump up against her. She doesn’t like socks with a heel or toe seam or any tags on clothes. Why is Travis always chewing? Why doesn’t Sierra liked to be touched? Why do children react differently to things around them? These children have different ways of reacting to the things around them, to sensations. Over the years, different terms (such as sensory integration) have been used to describe how children deal with the information they receive through their senses. Currently, the term being used to describe children who have difficulty dealing with input from their senses is sensory processing disorder. _____________________________________________________________________ Sensory Processing Disorder -

Extraocular Muscles Orbital Muscles
EXTRAOCULAR MUSCLES ORBITAL MUSCLES INTRA- EXTRA- OCULAR OCULAR CILIARY MUSCLES INVOLUNTARY VOLUNTARY 1.Superior tarsal muscle. 1.Levator Palpebrae Superioris 2.Inferior tarsal muscle 2.Superior rectus 3.Inferior rectus 4.Medial rectus 5.Lateral rectus 6.Superior oblique 7.Inferior oblique LEVATOR PALPEBRAE SUPERIORIOS Origin- Inferior surface of lesser wing of sphenoid. Insertion- Upper lamina (Voluntary) - Anterior surface of superior tarsus & skin of upper eyelid. Middle lamina (Involuntary) - Superior margin of superior tarsus. (Superior Tarsus Muscle / Muller muscle) Lower lamina (Involuntary) - Superior conjunctival fornix Nerve Supply :- Voluntary part – Oculomotor Nerve Involuntary part – Sympathetic ACTION :- Elevation of upper eye lid C/S :- Drooping of upper eyelid. Congenital ptosis due to localized myogenic dysgenesis Complete ptosis - Injury to occulomotor nerve. Partial ptosis - disruption of postganglionic sympathetic fibres from superior cervical sympathetic ganglion. Extra ocular Muscles : Origin Levator palpebrae superioris Superior Oblique Superior Rectus Lateral Rectus Medial Rectus Inferior Oblique Inferior Rectus RECTUS MUSCLES : ORIGIN • Arises from a common tendinous ring knows as ANNULUS OF ZINN • Common ring of connective tissue • Anterior to optic foramen • Forms a muscle cone Clinical Significance Retrobulbar neuritis ○ Origin of SUPERIOR AND MEDIAL RECTUS are closely attached to the dural sheath of the optic nerve, which leads to pain during upward & inward movements of the globe. Thyroid orbitopathy ○ Medial & Inf.rectus thicken. especially near the orbital apex - compression of the optic nerve as it enters the optic canal adjacent to the body of the sphenoid bone. Ophthalmoplegia ○ Proptosis occur due to muscle laxity. Medial Rectus Superior Rectus Origin :- Superior limb of the tendonous ring, and optic nerve sheath. -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -

A Real-Time Retinomorphic Simulator Using a Conductance-Based Discrete Neuronal Network
A Real-Time Retinomorphic Simulator Using a Conductance-Based Discrete Neuronal Network Seungbum Baek1;5, Jason K. Eshraghian2;5, Wesley Thio2, Yulia Sandamirskaya3, Herbert H.C. Iu 4 and Wei D. Lu2 1College of Electrical and Computer Engineering, Chungbuk National University, Cheongju 362763, South Korea 2School of Electrical, Electronic and Computer Engineering, University of Michigan, Ann Arbor, MI 48109 USA 3Institute of Neuroinformatics Neuroscience Center Zurich University and ETH Zurich, Switzerland 4School of Electrical, Electronic and Computer Engineering, University of Western Australia, Crawley, WA 6009, Australia 5These authors contributed equally to this manuscript. Abstract—We present an optimized conductance-based retina [2], and fabricating high-performance image sensors that are microcircuit simulator which transforms light stimuli into a on par with the specifications of the retina in terms of power series of graded and spiking action potentials through photo dissipation, dynamic range, and resolution [3]–[7]. At present, transduction. We use discrete retinal neuron blocks based on a collation of single-compartment models and morphologically most bio-inspired image processors trade-off the ability to pass realistic formulations, and successfully achieve a biologically low-frequency content and low spatial resolution, in favor of real-time simulator. This is done by optimizing the numerical practicality. methods employed to solve the system of over 270 nonlinear Beyond hardware, a similar distinction exists between bi- -
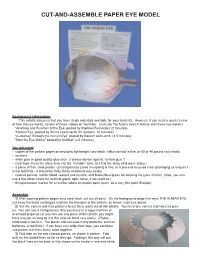
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

The Visual System: Higher Visual Processing
The Visual System: Higher Visual Processing Primary visual cortex The primary visual cortex is located in the occipital cortex. It receives visual information exclusively from the contralateral hemifield, which is topographically represented and wherein the fovea is granted an extended representation. Like most cortical areas, primary visual cortex consists of six layers. It also contains, however, a prominent stripe of white matter in its layer 4 - the stripe of Gennari - consisting of the myelinated axons of the lateral geniculate nucleus neurons. For this reason, the primary visual cortex is also referred to as the striate cortex. The LGN projections to the primary visual cortex are segregated. The axons of the cells from the magnocellular layers terminate principally within sublamina 4Ca, and those from the parvocellular layers terminate principally within sublamina 4Cb. Ocular dominance columns The inputs from the two eyes also are segregated within layer 4 of primary visual cortex and form alternating ocular dominance columns. Alternating ocular dominance columns can be visualized with autoradiography after injecting radiolabeled amino acids into one eye that are transported transynaptically from the retina. Although the neurons in layer 4 are monocular, neurons in the other layers of the same column combine signals from the two eyes, but their activation has the same ocular preference. Bringing together the inputs from the two eyes at the level of the striate cortex provide a basis for stereopsis, the sensation of depth perception provided by binocular disparity, i.e., when an image falls on non-corresponding parts of the two retinas. Some neurons respond to disparities beyond the plane of fixation (far cells), while others respond to disparities in front of the plane of the fixation (near cells).