The Role of the Sclera and Orbital Tissues in the Biomechanical Deformation Response of the Cornea and Whole Eye Under Loading B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

Symptoms of Age Related Macular Degeneration
WHAT IS MACULAR DEGENERATION? wavy or crooked, visual distortions, doorway and the choroid are interrupted causing waste or street signs seem bowed, or objects may deposits to form. Lacking proper nutrients, the light- Age related macular degeneration (AMD) is appear smaller or farther away than they sensitive cells of the macula become damaged. a disease that may either suddenly or gradually should, decrease in or loss of central vision, and The damaged cells can no longer send normal destroy the macula’s ability to maintain sharp, a central blurry spot. signals from the macula through the optic nerve to central vision. Interestingly, one’s peripheral or DRY: Progression with dry AMD is typically slower your brain, and consequently your vision becomes side vision remains unaffected. AMD is the leading de-gradation of central vision: need for increasingly blurred cause of “legal blindness” in the United States for bright illumination for reading or near work, diffi culty In either form of AMD, your vision may remain fi ne persons over 65 years of age. AMD is present in adapting to low levels of illumination, worsening blur in one eye up to several years even while the other approximately 10 percent of the population over of printed words, decreased intensity or brightness of eye’s vision has degraded. Most patients don’t the age of 52 and in up to 33 percent of individuals colors, diffi culty recognizing faces, gradual increase realize that one eye’s vision has been severely older than 75. The macula allows alone gives us the in the haziness of overall vision, and a profound drop reduced because your brain compensates the bad ability to have: sharp vision, clear vision, color vision, in your central vision acuity. -

Cultivating a Cure for Blindness
news and views were taken in a l-mm2 biopsy from the good eye, then they were cultured and, on second Cultivating a cure passage, they formed a tightly packed and communicating (confluent) monolayer of cells. For grafting, the conjunctiva! epi for blindness thelium was completely removed from the cornea and limbus of the recipient eye, and replaced with a slightly larger monolayer of Stuart Hodson cultured limbal epithelium. The eyes were Damaged corneas can often be repaired using donor grafts, but if the then covered with therapeutic soft contact damage is too great the graft wlll be rejected. This may change with the lenses, and tightly patched for several days. development of a method to Inhibit rejection which uses cultivated cells. The results after the limbal epithelial graft were very promising. The grafted epithelium he human cornea has a special proper epithelium can mould a smooth apical and was stable, transparent, multi-layered and Tty known as immune privilege, which basal surface, the limbal epithelial stem cells smooth. One of the patients had suffered an allows tissue grafts from donors to be do not form such a smooth surface. alkali burn to his left eye ten years earlier, and carried out without the usual problems of If the corneal epithelium is lost it can be had undergone three previous unsuccessful immune rejection. However, immune privi functionally regenerated by the limbal stem corneal grafts. Before the treatment he had lege is lost at the perimeter of the cornea - cells. But if both the corneal and limbal continual severe corneal vascularization the limb us of the eye - where the transpar epithelia are lost, the corneal surface is re ( development of blood vessels) and persis ent corneal stroma meets the opaque sclera colonized by the other neighbour of the tent ulceration, and the eye was painful and (Fig. -

Quantitative Assessment of Central and Limbal Epithelium After Long
Eye (2016) 30, 979–986 © 2016 Macmillan Publishers Limited All rights reserved 0950-222X/16 www.nature.com/eye 1,5 1,5 1 Quantitative RK Prakasam , BS Kowtharapu , K Falke , CLINICAL STUDY K Winter2,3, D Diedrich4, A Glass4, A Jünemann1, assessment of central RF Guthoff1 and O Stachs1 and limbal epithelium after long-term wear of soft contact lenses and in patients with dry eyes: a pilot study Abstract Purpose Analysis of microstructural Eye (2016) 30, 979–986; doi:10.1038/eye.2016.58; alterations of corneal and limbal epithelial published online 22 April 2016 cells in healthy human corneas and in other ocular conditions. Introduction Patients and methods Unilateral eyes of three groups of subjects include healthy The X, Y, Z hypothesis1 explains cell mechanism volunteers (G1, n = 5), contact lens wearers that is essential for the renewal and maintenance 1Department of (G2, n = 5), and patients with dry eyes of the corneal epithelium. This hypothesis Ophthalmology, University = proposes that the loss of corneal epithelial of Rostock, Rostock, (G3, n 5) were studied. Imaging of basal Germany (BC) and intermediate (IC) epithelial cells surface cells (Z) can be maintained by the from central cornea (CC), corneal limbus proliferation of basal epithelial cells (X), and the 2Faculty of Medicine, centripetal movements of the peripheral (CL) and scleral limbus (SL) was obtained by Institute of Anatomy, epithelial cells (Y). By utilizing this mechanism, University of Leipzig, in vivo confocal microscopy (IVCM). An it is also possible to categorize both disease and Leipzig, Germany appropriate image analysis algorithm was therapies according to the specific component 3 used to quantify morphometric parameters involved.1 Therefore it is vital to understand the Institute for Medical including mean cell area, compactness, Informatics, Statistics and cellular structures of both central and limbal Epidemiology (IMISE), solidity, major and minor diameter, and epithelial cells in normal and in various corneal University of Leipzig, maximum boundary distance. -

Development of in Vitro Corneal Models: Opportunity for Pharmacological Testing
Review Development of In Vitro Corneal Models: Opportunity for Pharmacological Testing Valentina Citi 1, Eugenia Piragine 1, Simone Brogi 1,* , Sara Ottino 2 and Vincenzo Calderone 1 1 Department of Pharmacy, University of Pisa, Via Bonanno 6, 56126 Pisa, Italy; [email protected] (V.C.); [email protected] (E.P.); [email protected] (V.C.) 2 Farmigea S.p.A., Via G.B. Oliva 6/8, 56121 Pisa, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-050-2219-613 Received: 24 October 2020; Accepted: 30 October 2020; Published: 2 November 2020 Abstract: The human eye is a specialized organ with a complex anatomy and physiology, because it is characterized by different cell types with specific physiological functions. Given the complexity of the eye, ocular tissues are finely organized and orchestrated. In the last few years, many in vitro models have been developed in order to meet the 3Rs principle (Replacement, Reduction and Refinement) for eye toxicity testing. This procedure is highly necessary to ensure that the risks associated with ophthalmic products meet appropriate safety criteria. In vitro preclinical testing is now a well-established practice of significant importance for evaluating the efficacy and safety of cosmetic, pharmaceutical, and nutraceutical products. Along with in vitro testing, also computational procedures, herein described, for evaluating the pharmacological profile of potential ocular drug candidates including their toxicity, are in rapid expansion. In this review, the ocular cell types and functionality are described, providing an overview about the scientific challenge for the development of three-dimensional (3D) in vitro models. -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -
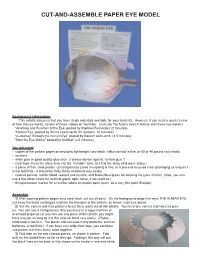
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -
The Orbit Is Composed Anteri
DAVID L. PARVER, MD The University of Texas Southwestern Medical Center, Dallas Theability to successfully assess and treat The Orbit physical ailments requires an understanding of the anatomy involved in the injury or The eye itself lies within a protective shell trauma. When dealing with injuries and called the bony orbits. These bony cavities are trauma associated with the eye, it is neces- located on each side of the root of the nose. sary to have a work- Each orbit is structured like a pear with the ing knowledge of optic nerve, the nerve that carries visual im- basic ocular anatomy pulses from the retina to the brain, represent- so that an accurate ing the stem of the orbtt (Duke-Elder, 1976). Understa eye also diagnosis can be Seven bones make up the bony orbit: frontal, achieved and treat- zygomatic, maxillary, ethmoidal, sphenoid, ment can be imple- lacrimal, and palatine (Figures 1 and 2). in a bony " mented. The roof of the orbit is composed anteri- . .. The upcoming ar- orly of the orbital plate of the frontal bone ticles in this special and posteriorly by the lesser wing of the sphe- Each portion of the 01 I noid bone. The lateral wall is separated from .r. theme section the nervc an eye will deal specifically 2 with recognizing ocular illness, disease, and injuries, and will also address the incidence of sports related eye injuries and trauma. This paper covers the ba- sics of eye anatomy, focusing on the eye globe and its surrounding struc- tures. Once one gains an understand- ing of the normal anatomy of the eye, it will be easier to recognize trauma, injury, or illness. -

Scleral Lenses and Eye Health
Scleral Lenses and Eye Health Anatomy and Function of the Human Eye How Scleral Lenses Interact with the Ocular Surface Just as the skin protects the human body, the ocular surface protects the human Scleral lenses are large-diameter lenses designed to vault the cornea and rest on the conjunctival tissue sitting on eye. The ocular surface is made up of the cornea, the conjunctiva, the tear film, top of the sclera. The space between the back surface of the lens and the cornea acts as a fluid reservoir. Scleral and the glands that produce tears, oils, and mucus in the tear film. lenses can range in size from 13mm to 19mm, although larger diameter lenses may be designed for patients with more severe eye conditions. Due to their size, scleral lenses consist SCLERA: The sclera is the white outer wall of the eye. It is SCLERAL LENS made of collagen fibers that are arranged for strength rather of at least two zones: than transmission of light. OPTIC ZONE The optic zone vaults over the cornea CORNEA: The cornea is the front center portion of the outer Cross section of FLUID RESERVOIR wall of the eye. It is made of collagen fibers that are arranged in the eye shows The haptic zone rests on the conjunctiva such a way so that the cornea is clear. The cornea bends light the cornea, overlying the sclera as it enters the eye so that the light is focused on the retina. conjunctiva, and sclera as CORNEA The cornea has a protective surface layer called the epithelium. -

Evisceration, Enucleation and Exenteration
CHAPTER 10 EVISCERATION, ENUCLEATION AND EXENTERATION This chapter describes three operations that either remove the contents of the eye (evisceration), the eye itself (enucleation) or the whole orbital contents (exenteration). Each operation has specific indications which are important to understand. In many cultures the removal of an eye, even if blind, is resisted. If an eye is very painful or grossly disfigured an operation will be accepted more readily. However, if the eye looks normal the patient or their family may be very reluctant to accept its removal. Therefore tact, compassion and patience are needed when recommending these operations. ENUCLEATION AND EVISCERATION There are several reasons why either of these destructive operations may be necessary: 1. Malignant tumours in the eye. In the case of a malignant tumour or suspected malignant tumour the eye should be removed by enucleation and not evisceration.There are two important intraocular tumours, retinoblastoma and melanoma and for both of them the basic treatment is enucleation. Retinoblastoma is a relatively common tumour in early childhood. At first the growth is confined to the eye. Enucleation must be carried out at this stage and will probably save the child’s life. It is vital not to delay or postpone surgery. If a child under 6 has a blind eye and the possibility of a tumour cannot be ruled out, it is best to remove the eye. Always examine the other eye very carefully under anaesthetic as well. It may contain an early retinoblastoma which could be treatable and still save the eye. Retinoblastoma spreads along the optic nerve to the brain. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst.