Ballast Water Pathogens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Euphyllia Paradivisa :: Biological Information
LISTED CORALS IN THE INDO-PACIFIC Euphyllia paradivisa :: Biological Information MORPHOLOGY Pacific Islands Region Colonies of Euphyllia paradivisa are made up of branching, separate corallites. Polyps have branching tentacles. Color is pale greenish-grey or pink (in rare instances) with lighter tentacle tips. Photos copyright: J.E.N. Veron (left), Douglas Fenner (right) REPRODUCTION Euphyllia paradivisa’s reproductive mode is not known. Other Euphyllia species display a variety of reproductive modes so it is unclear which is most probable of this species. :: Spatial Information GEOGRAPHIC RANGE Based on confrmed observations and strong predictions of occurrence in areas that have not yet been surveyed sufciently, Euphyllia paradivisa is likely distributed mostly in the Coral Triangle area (the Philippines to Timor Leste and east to the Solomon Islands). It is also confrmed to occur in American Samoa. For more information contact: NMFS Pacifc Islands Regional Offce 1845 Wasp Blvd., Bldg. 176 Honolulu, HI 96818 Tel: 808-725-5000 Website: www.fpir.noaa.gov U.S. Department of Commerce | National Oceanic and Atmospheric Administration | NOAA Fisheries NOAA Fisheries | Listed Corals in the Indo-Pacific:Euphyllia paradivisa LEGEND Region with confrmed record of species occurrence Region with predicted record of species occurrence Region with published record of species occurrence that needs further investigation Region with no record of species occurrence Veron JEN, Stafford-Smith MG, Turak E and DeVantier LM (in prep.) Corals of the World www.coralsoftheworld.com OCCURRENCE IN U.S. JURISDICTIONS Euphyllia paradivisa has not yet been reported from Guam, the Commonwealth of the Northern Mariana Islands (CNMI), and the Pacifc Remote Island Areas (PRIA). -

Response of Fluorescence Morphs of the Mesophotic Coral Euphyllia Paradivisa to Ultra-Violet Radiation
www.nature.com/scientificreports OPEN Response of fuorescence morphs of the mesophotic coral Euphyllia paradivisa to ultra-violet radiation Received: 23 August 2018 Or Ben-Zvi 1,2, Gal Eyal 1,2,3 & Yossi Loya 1 Accepted: 15 March 2019 Euphyllia paradivisa is a strictly mesophotic coral in the reefs of Eilat that displays a striking color Published: xx xx xxxx polymorphism, attributed to fuorescent proteins (FPs). FPs, which are used as visual markers in biomedical research, have been suggested to serve as photoprotectors or as facilitators of photosynthesis in corals due to their ability to transform light. Solar radiation that penetrates the sea includes, among others, both vital photosynthetic active radiation (PAR) and ultra-violet radiation (UVR). Both types, at high intensities, are known to have negative efects on corals, ranging from cellular damage to changes in community structure. In the present study, fuorescence morphs of E. paradivisa were used to investigate UVR response in a mesophotic organism and to examine the phenomenon of fuorescence polymorphism. E. paradivisa, although able to survive in high-light environments, displayed several physiological and behavioral responses that indicated severe light and UVR stress. We suggest that high PAR and UVR are potential drivers behind the absence of this coral from shallow reefs. Moreover, we found no signifcant diferences between the diferent fuorescence morphs’ responses and no evidence of either photoprotection or photosynthesis enhancement. We therefore suggest that FPs in mesophotic corals might have a diferent biological role than that previously hypothesized for shallow corals. Te solar radiation that reaches the earth’s surface includes, among others, ultra-violet radiation (UVR; 280– 400 nm) and photosynthetically active radiation (PAR; 400–700 nm). -

Resurrecting a Subgenus to Genus: Molecular Phylogeny of Euphyllia and Fimbriaphyllia (Order Scleractinia; Family Euphylliidae; Clade V)
Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphylliidae; clade V) Katrina S. Luzon1,2,3,*, Mei-Fang Lin4,5,6,*, Ma. Carmen A. Ablan Lagman1,7, Wilfredo Roehl Y. Licuanan1,2,3 and Chaolun Allen Chen4,8,9,* 1 Biology Department, De La Salle University, Manila, Philippines 2 Shields Ocean Research (SHORE) Center, De La Salle University, Manila, Philippines 3 The Marine Science Institute, University of the Philippines, Quezon City, Philippines 4 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan 5 Department of Molecular and Cell Biology, James Cook University, Townsville, Australia 6 Evolutionary Neurobiology Unit, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan 7 Center for Natural Sciences and Environmental Research (CENSER), De La Salle University, Manila, Philippines 8 Taiwan International Graduate Program-Biodiversity, Academia Sinica, Taipei, Taiwan 9 Institute of Oceanography, National Taiwan University, Taipei, Taiwan * These authors contributed equally to this work. ABSTRACT Background. The corallum is crucial in building coral reefs and in diagnosing systematic relationships in the order Scleractinia. However, molecular phylogenetic analyses revealed a paraphyly in a majority of traditional families and genera among Scleractinia showing that other biological attributes of the coral, such as polyp morphology and reproductive traits, are underutilized. Among scleractinian genera, the Euphyllia, with nine nominal species in the Indo-Pacific region, is one of the groups Submitted 30 May 2017 that await phylogenetic resolution. Multiple genetic markers were used to construct Accepted 31 October 2017 Published 4 December 2017 the phylogeny of six Euphyllia species, namely E. ancora, E. divisa, E. -

Final Corals Supplemental Information Report
Supplemental Information Report on Status Review Report And Draft Management Report For 82 Coral Candidate Species November 2012 Southeast and Pacific Islands Regional Offices National Marine Fisheries Service National Oceanic and Atmospheric Administration Department of Commerce Table of Contents INTRODUCTION ............................................................................................................................................. 1 Background ............................................................................................................................................... 1 Methods .................................................................................................................................................... 1 Purpose ..................................................................................................................................................... 2 MISCELLANEOUS COMMENTS RECEIVED ...................................................................................................... 3 SRR EXECUTIVE SUMMARY ........................................................................................................................... 4 1. Introduction ........................................................................................................................................... 4 2. General Background on Corals and Coral Reefs .................................................................................... 4 2.1 Taxonomy & Distribution ............................................................................................................. -

Tonga (866) 874-7639 (855) 225-8086
American Ingenuity www.livestockusa.org Tonga (866) 874-7639 (855) 225-8086 Wednesday to LAX, Thursday to you Tranship - F.O.B. Tonga Order Cut-off is on Thursdays! Animal cost plus landing costs "Poor man's Australia!" See landing costs below Acros, Montis, and Euphys are especially awesome! Demand is very heavy, order early! August 11, 2021 Note: List comes to us directly from Tonga Next shipment will be mid-late August Order Code Genus or binomial Common Name Cost HARD CORALS CH-ACANAS Acanthastrea echinata Acanthastrea $41.00 CH-ACANAS Acanthastrea Bowerbanki Acanthastrea $55.00 CH-ACROGR Acropora sp Green Acropora $55.00 CH-ACROPK Acropora sp Pink Acropora Millepora $55.00 CH-ACROPU Acropora sp Purple Acropora $55.00 CH-ACROYL Acropora sp Yellow Acropora $55.00 CH-ACROBL Acropora sp Blue Acropora $55.00 CH-ACROTR Acropora sp Tri-colored Acropora $55.00 CH-ASTRAS Astreopora sp Astreopora $27.00 CH-BLASTO Blastomussa sp Blastomussa $60.00 CH-CAULAS Caulastrea sp Candycane Coral $27.00 CH-CYNAAS Cynarina Lacrymalis Button Coral $37.50 CH-CYPHAS Cyphastrea sp Cyphastrea $27.00 CH-ECHINO Echinophyllia sp Echinophyllia $55.00 CH-EUPHHM Euphyllia Cristata Torch Coral - Grape $55.00 CH-EUPHGT Euphyllia paradivisa Frogspawn $50.00 CH-EUPHYT Euphyllia paradivisa Frogspawn $50.00 CH-EUPHAT Euphyllia Glabrescens Torch Coral $50.00 CH-EUPHMG Euphyllia Glabrescens Torch Coral - Green Metallic $55.00 Euphyllia GOLD TORCH ( Available sometimes ) $90.00 CH-EUPHPAR Euphyllia Paranacora Hammer Coral $58.00 CH-FAVYEL Favia sp Favia - Yellow $27.00 CH-FAVCOL -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of -

Yossi Loya Ph
Yossi Loya SELECTED PUBLICATIONS (Journals) For full list see: http://en-lifesci.tau.ac.il/profile/yosiloya 5. Loya Y. (1972). Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Mar. Biol. 13: 100-123. DOI: 10.1007/BF00366561. 6. Loya Y. (1975). Possible effects of water pollution on the community structure of Red Sea corals. Mar. Biol. 29: 177-185. DOI: 10.1007/BF00388987 9. Loya Y. (1976a). The Red Sea coral Stylophora pistillata is an r-strategist. Nature 259: 478-480.DOI: 10.1038/259478a0 10. Loya Y. (1976b). Recolonization of Red Sea corals affected by natural catastrophes and man- made perturbations. Ecology 57: 278-289 DOI:10.2307/1934816. 11. Loya Y. (l976c). Effects of water turbidity and sedimentation on community structure of Puerto Rican corals. Bull. Mar. Sci. 26: 450-466. 12. Loya Y. (1976d). Skeletal regeneration rate in a Red Sea scleractinian coral population. Nature 261: 490-491 261:490-491 DOI: 10.1038/261490a0. 20. Bradbury R.H., Y. Loya (1978). A heuristic analysis of spatial patterns of hermatypic corals at Eilat, Red Sea. American Naturalist, 112:493-507. DOI: 10.1086/283292 22. Rinkevich B. & Y. Loya (1979a). The reproduction of the Red Sea coral Stylophora pistillata. I. Gonads and planulae. Mar. Ecol. Prog. Ser. 2: 133-144. DOI: 10.3354/meps001145. 23. Rinkevich B. & Y. Loya (1979b).The reproduction of the Red Sea coral Stylophora pistillata.II. Synchronization in breeding and seasonality of planulae shedding. MEPS 2: 145-152 DOI: 10.3354/meps001145. 27. Loya Y. & B. Rinkevich (1980).Effects of oil pollution on coral reef communities. -
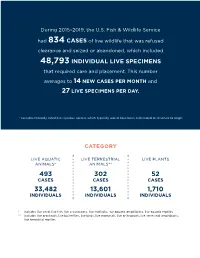
Seizure Data Report
During 2015–2019, the U.S. Fish & Wildlife Service had 834 CASES of live wildlife that was refused clearance and seized or abandoned, which included 48,793 INDIVIDUAL LIVE SPECIMENS that required care and placement. This number averages to 14 NEW CASES PER MONTH and 27 LIVE SPECIMENS PER DAY. * excludes federally listed live injurious species which typically would have been euthanized or returned to origin CATEGORY LIVE AQUATIC LIVE TERRESTRIAL LIVE PLANTS ANIMALS* ANIMALS** 493 302 52 CASES CASES CASES 33,482 13,601 1,710 INDIVIDUALS INDIVIDUALS INDIVIDUALS * includes live coral, live fish, live crustaceans, live mollusks, live aquatic amphibians, live aquatic reptiles ** includes live arachnids, live butterflies, live birds, live mammals, live arthropods, live terrestrial amphibians, live terrestrial reptiles CASES Note: Some cases involved multiple wildlife categories, and/or a combination of terrestrial and aquatic species. Therefore, the accumulative number of cases depicted above and below (848) will exceed the total number of cases above (834). Percentages below are representative of the percentage value of total number of cases (834). LIVE REPTILE SPECIES were the most frequently confiscated taxa, by number of cases: 244 cases (~29% of total cases) The TOP THREE taxa by number of cases were: REPTILES CORAL FISH 244 210 137 (~29% of total cases) (~25% of total cases) (~17% of total cases) The remaining breakdown of taxa by the number of cases were: BIRDS CRUSTACEANS ARACHNIDS CACTUS 69 42 35 30 (~8% of total cases) (~5% of total -

Coral Reef Species List
Coral Reef Gallery The Philippine Coral Reef Tank focuses on the most diverse and fragile of marine ecosystems. From the main exhibit floor, visitors look down on a shallow, sandy lagoon—a calm, protected area inhabited by sharks, rays, and colorful fishes. Where the lagoon drops off to the deep reef, hundreds of bright fishes visible near the surface lure the visitor to view the spectacle one floor below. There, dramatic underwater views of the deep reef invite contemplation. Featuring 1,000 square feet of living coral and some 4,000 fish of 100 or more species, this 212,000‐gallon exhibit is, at 25 feet, the deepest and one of the largest displays of a living coral reef in the world. Curiosity leads to exploration of several smaller galleries along the perimeter of the exhibit that highlight the unique adaptations and complex interactions of reef organisms. Acanthastrea echinata Acanthurus achilles Acanthurus blochii Acanthurus coeruleus Blue tang Acanthurus dussumieri Acanthurus japonicus Acanthurus lineatus Acanthurus mata Acanthurus nigricans Acanthurus nigrofuscus Acanthurus nigroris Acanthurus olivaceus Acanthurus pyroferus Acanthurus triostegus Acanthurus xanthopterus Acropora formosa Acropora gemmifera Acropora micropthalma Acropora millepora Acropora sp. Staghorn Coral Acropora youngei Aeoliscus strigatus Shrimpfish Alcyonium sp. Alpheus randalli Randall’s Partner Shrimp Alveopora sp. Ambligobius hectori Hector’s Goby Ambligobius rainfordi Rainford’s Goby Amblycirrhitus pinos Redspotted hawkfish Amblyeleotris randalli Randall’s -

Center for Biological Diversity-2009-TN1518-Ctr Bio
BEFORE THE SECRETARY OF COMMERCE PETITION TO LIST 83 CORAL SPECIES UNDER THE ENDANGERED SPECIES ACT Blue rice coral photo © Keoki Stender Submitted October 20, 2009 NOTICE OF PETITION Gary Locke Secretary of Commerce U.S. Department of Commerce 1401 Constitution Avenue, N.W., Room 5516 Washington, D.C. 20230 E-mail: [email protected] James Balsiger, Acting Director NOAA Fisheries National Oceanographic and Atmospheric Administration 1315 East-West Highway Silver Springs, MD 20910 E-mail: [email protected] PETITIONER The Center for Biological Diversity 351 California Street, Suite 600 San Francisco, CA 94104 ph: (415) 436-9682 fax: (415) 436-9683 Date: October 20, 2009 Miyoko Sakashita Shaye Wolf Center for Biological Diversity Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. §424.14(a), the Center for Biological Diversity (“Petitioner”) hereby petitions the Secretary of Commerce and the National Oceanographic and Atmospheric Administration (“NOAA”), through the National Marine Fisheries Service (“NMFS” or “NOAA Fisheries”), to list 83 coral species and to designate critical habitat to ensure their survival and recovery. The Center for Biological Diversity (“Center”) is a non-profit, public interest environmental organization dedicated to the protection of native species and their habitats through science, policy, and environmental law. The Center has over 43,000 members throughout the United States and internationally. The Center and its members are concerned with the conservation of endangered species, including coral species, and the effective implementation of the ESA. -

Yossi Loya Ph
Sept. 2019 Curriculum vitae Yossi Loya Professor of Marine Biology School of Zoology, Tel Aviv University Tel Aviv, 69978 Israel Lab Tel: 972-3-640-7683 Fax: 972-3-640-7682 E-mail: [email protected] http://en-lifesci.tau.ac.il/profile/yosiloya http://scholar.google.co.il/citations?user=cpv4vqAAAAAJ http://www.academy.ac.il/asp/members/members_in.asp?person_id=1156 Home 31 Hakochav st. Raanana 43568 Tel: 972-9-774-1921 Fax: 972-9-771-6429 Mobile: 054-637-2121 1 Yossi Loya Tel Aviv University Date of birth:…………...23 May, 1942 Place of birth:…………..Plovdiv, Bulgaria Year of immigration…..1944 Zahal, Military Service: 1960-1962 Marital Status:................Married to Shoshana Loya Children: Yael, Shay and Assaf EDUCATION Year University/ Institute Department Degree 1962-1965 Tel Aviv University, Israel Biology B.Sc. 1965-1967 Tel Aviv University, Israel Zoology M.Sc. 1967-1971 State University of New York at Stony Brook, L.I. N.Y. Ecology Ph.D. M.Sc. Thesis: Ecology of fish breeding in brackish water ponds near the Dead Sea (Suma cum laude). Ph.D. Thesis: Community structure and species diversity hermatypic corals at Eilat, Red Sea. Supervisor: Prof. L.B. Slobodkin Post doctorate Woods Hole Oceanographic Institution, Mass. 1971-1972 Research: Oil pollution effects on benthic communities in Buzzards Bay Woods Hole- Supervisor: Prof. Howard Sanders AREAS OF SCIENTIFIC INTEREST Ecology and Evolution of reef corals; Coral reef community structure; Species diversity and community structure of corals; Life history strategies of reef corals