Response of Fluorescence Morphs of the Mesophotic Coral Euphyllia Paradivisa to Ultra-Violet Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

SEDIMENTARY FRAMEWORK of Lmainland FRINGING REEF DEVELOPMENT, CAPE TRIBULATION AREA
GREAT BARRIER REEF MARINE PARK AUTHORITY TECHNICAL MEMORANDUM GBRMPA-TM-14 SEDIMENTARY FRAMEWORK OF lMAINLAND FRINGING REEF DEVELOPMENT, CAPE TRIBULATION AREA D.P. JOHNSON and RM.CARTER Department of Geology James Cook University of North Queensland Townsville, Q 4811, Australia DATE November, 1987 SUMMARY Mainland fringing reefs with a diverse coral fauna have developed in the Cape Tribulation area primarily upon coastal sedi- ment bodies such as beach shoals and creek mouth bars. Growth on steep rocky headlands is minor. The reefs have exten- sive sandy beaches to landward, and an irregular outer margin. Typically there is a raised platform of dead nef along the outer edge of the reef, and dead coral columns lie buried under the reef flat. Live coral growth is restricted to the outer reef slope. Seaward of the reefs is a narrow wedge of muddy, terrigenous sediment, which thins offshore. Beach, reef and inner shelf sediments all contain 50% terrigenous material, indicating the reefs have always grown under conditions of heavy terrigenous influx. The relatively shallow lower limit of coral growth (ca 6m below ADD) is typical of reef growth in turbid waters, where decreased light levels inhibit coral growth. Radiocarbon dating of material from surveyed sites confirms the age of the fossil coral columns as 33304110 ybp, indicating that they grew during the late postglacial sea-level high (ca 5500-6500 ybp). The former thriving reef-flat was killed by a post-5500 ybp sea-level fall of ca 1 m. Although this study has not assessed the community structure of the fringing reefs, nor whether changes are presently occur- ring, it is clear the corals present today on the fore-reef slope have always lived under heavy terrigenous influence, and that the fossil reef-flat can be explained as due to the mid-Holocene fall in sea-level. -

Euphyllia Paradivisa :: Biological Information
LISTED CORALS IN THE INDO-PACIFIC Euphyllia paradivisa :: Biological Information MORPHOLOGY Pacific Islands Region Colonies of Euphyllia paradivisa are made up of branching, separate corallites. Polyps have branching tentacles. Color is pale greenish-grey or pink (in rare instances) with lighter tentacle tips. Photos copyright: J.E.N. Veron (left), Douglas Fenner (right) REPRODUCTION Euphyllia paradivisa’s reproductive mode is not known. Other Euphyllia species display a variety of reproductive modes so it is unclear which is most probable of this species. :: Spatial Information GEOGRAPHIC RANGE Based on confrmed observations and strong predictions of occurrence in areas that have not yet been surveyed sufciently, Euphyllia paradivisa is likely distributed mostly in the Coral Triangle area (the Philippines to Timor Leste and east to the Solomon Islands). It is also confrmed to occur in American Samoa. For more information contact: NMFS Pacifc Islands Regional Offce 1845 Wasp Blvd., Bldg. 176 Honolulu, HI 96818 Tel: 808-725-5000 Website: www.fpir.noaa.gov U.S. Department of Commerce | National Oceanic and Atmospheric Administration | NOAA Fisheries NOAA Fisheries | Listed Corals in the Indo-Pacific:Euphyllia paradivisa LEGEND Region with confrmed record of species occurrence Region with predicted record of species occurrence Region with published record of species occurrence that needs further investigation Region with no record of species occurrence Veron JEN, Stafford-Smith MG, Turak E and DeVantier LM (in prep.) Corals of the World www.coralsoftheworld.com OCCURRENCE IN U.S. JURISDICTIONS Euphyllia paradivisa has not yet been reported from Guam, the Commonwealth of the Northern Mariana Islands (CNMI), and the Pacifc Remote Island Areas (PRIA). -

Resurrecting a Subgenus to Genus: Molecular Phylogeny of Euphyllia and Fimbriaphyllia (Order Scleractinia; Family Euphylliidae; Clade V)
Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphylliidae; clade V) Katrina S. Luzon1,2,3,*, Mei-Fang Lin4,5,6,*, Ma. Carmen A. Ablan Lagman1,7, Wilfredo Roehl Y. Licuanan1,2,3 and Chaolun Allen Chen4,8,9,* 1 Biology Department, De La Salle University, Manila, Philippines 2 Shields Ocean Research (SHORE) Center, De La Salle University, Manila, Philippines 3 The Marine Science Institute, University of the Philippines, Quezon City, Philippines 4 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan 5 Department of Molecular and Cell Biology, James Cook University, Townsville, Australia 6 Evolutionary Neurobiology Unit, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan 7 Center for Natural Sciences and Environmental Research (CENSER), De La Salle University, Manila, Philippines 8 Taiwan International Graduate Program-Biodiversity, Academia Sinica, Taipei, Taiwan 9 Institute of Oceanography, National Taiwan University, Taipei, Taiwan * These authors contributed equally to this work. ABSTRACT Background. The corallum is crucial in building coral reefs and in diagnosing systematic relationships in the order Scleractinia. However, molecular phylogenetic analyses revealed a paraphyly in a majority of traditional families and genera among Scleractinia showing that other biological attributes of the coral, such as polyp morphology and reproductive traits, are underutilized. Among scleractinian genera, the Euphyllia, with nine nominal species in the Indo-Pacific region, is one of the groups Submitted 30 May 2017 that await phylogenetic resolution. Multiple genetic markers were used to construct Accepted 31 October 2017 Published 4 December 2017 the phylogeny of six Euphyllia species, namely E. ancora, E. divisa, E. -

Final Corals Supplemental Information Report
Supplemental Information Report on Status Review Report And Draft Management Report For 82 Coral Candidate Species November 2012 Southeast and Pacific Islands Regional Offices National Marine Fisheries Service National Oceanic and Atmospheric Administration Department of Commerce Table of Contents INTRODUCTION ............................................................................................................................................. 1 Background ............................................................................................................................................... 1 Methods .................................................................................................................................................... 1 Purpose ..................................................................................................................................................... 2 MISCELLANEOUS COMMENTS RECEIVED ...................................................................................................... 3 SRR EXECUTIVE SUMMARY ........................................................................................................................... 4 1. Introduction ........................................................................................................................................... 4 2. General Background on Corals and Coral Reefs .................................................................................... 4 2.1 Taxonomy & Distribution ............................................................................................................. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -

De Novo Transcriptome Assembly from the Gonads of a Scleractinian Coral
Chiu et al. BMC Genomics (2020) 21:732 https://doi.org/10.1186/s12864-020-07113-9 RESEARCH ARTICLE Open Access De novo transcriptome assembly from the gonads of a scleractinian coral, Euphyllia ancora: molecular mechanisms underlying scleractinian gametogenesis Yi-Ling Chiu1,2, Shinya Shikina3,4*, Yuki Yoshioka5, Chuya Shinzato5* and Ching-Fong Chang4,6* Abstract Background: Sexual reproduction of scleractinians has captured the attention of researchers and the general public for decades. Although extensive ecological data has been acquired, underlying molecular and cellular mechanisms remain largely unknown. In this study, to better understand mechanisms underlying gametogenesis, we isolated ovaries and testes at different developmental phases from a gonochoric coral, Euphyllia ancora, and adopted a transcriptomic approach to reveal sex- and phase-specific gene expression profiles. In particular, we explored genes associated with oocyte development and maturation, spermiogenesis, sperm motility / capacitation, and fertilization. Results: 1.6 billion raw reads were obtained from 24 gonadal samples. De novo assembly of trimmed reads, and elimination of contigs derived from symbiotic dinoflagellates (Symbiodiniaceae) and other organisms yielded a reference E. ancora gonadal transcriptome of 35,802 contigs. Analysis of 4 developmental phases identified 2023 genes that were differentially expressed during oogenesis and 678 during spermatogenesis. In premature/mature ovaries, 631 genes were specifically upregulated, with 538 in mature testes. Upregulated genes included those involved in gametogenesis, gamete maturation, sperm motility / capacitation, and fertilization in other metazoans, including humans. Meanwhile, a large number of genes without homology to sequences in the SWISS-PROT database were also observed among upregulated genes in premature / mature ovaries and mature testes. -

Symbiodinium—Invertebrate Symbioses and the Role of Metabolomics
Mar. Drugs 2010, 8, 2546-2568; doi:10.3390/md8102546 OPEN ACCESS Marine Drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Review Symbiodinium—Invertebrate Symbioses and the Role of Metabolomics Benjamin R. Gordon 1,* and William Leggat 2,3 1 AIMS@JCU, Australian Institute of Marine Science, School of Pharmacy and Molecular Sciences, James Cook University, Townsville, Queensland 4811, Australia 2 ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Queensland 4811, Australia; E-Mail: [email protected] 3 School of Pharmacy and Molecular Sciences, James Cook University, Townsville, Queensland 4811, Australia * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +61-7-47815395; Fax: +61-7-47816078. Received: 26 August 2010; in revised form: 24 September 2010 / Accepted: 26 September 2010 / Published: 30 September 2010 Abstract: Symbioses play an important role within the marine environment. Among the most well known of these symbioses is that between coral and the photosynthetic dinoflagellate, Symbiodinium spp. Understanding the metabolic relationships between the host and the symbiont is of the utmost importance in order to gain insight into how this symbiosis may be disrupted due to environmental stressors. Here we summarize the metabolites related to nutritional roles, diel cycles and the common metabolites associated with the invertebrate-Symbiodinium relationship. We also review the more obscure metabolites and toxins that have been identified through natural products and biomarker research. Finally, we discuss the key role that metabolomics and functional genomics will play in understanding these important symbioses. Keywords: metabolomics; zooxanthellae; marine; Symbiodinium; coral 1. -

Diel Patterns of Larval Release by Five Brooding Scleractinian Corals
MARINE ECOLOGY PROGRESS SERIES Vol. 321: 133–142, 2006 Published September 8 Mar Ecol Prog Ser Diel patterns of larval release by five brooding scleractinian corals Tung-Yung Fan1, 2,*, Ke-Han Lin1, 3, Fu-Wen Kuo1, Keryea Soong3, Li-Lian Liu3, Lee-Shing Fang1, 2 1National Museum of Marine Biology and Aquarium, Pingtung, Taiwan 944, ROC 2Institute of Marine Biodiversity and Evolution, National Dong Hwa University, Hualien, Taiwan 974, ROC 3Institute of Marine Biology, National Sun Yat-Sen University, Kaohsiung, Taiwan 804, ROC ABSTRACT: Timing of larval release in benthic marine invertebrates plays an important role in determining the reproductive success and extent of larval dispersal. Few studies have been conducted on diel variations in planula release by corals. Five brooding corals Seriatopora hystrix, Stylophora pistillata, Pocillopora damicornis, Euphyllia glabrescens, and Tubastraea aurea in Nan- wan Bay, southern Taiwan, were selected to compare diel patterns of planula release. Corals were collected from the field and maintained in outdoor, flow-through systems to quantify the hourly release of planulae. Planulation by S. hystrix and S. pistillata was highly synchronized with 1 peak of planula release occurring close to sunrise. Planulae of P. damicornis and E. glabrescens were released throughout the day, and usually 2 peaks occurred in the early morning and at night. Planu- lation of the ahermatypic coral T. aurea occurred throughout the day without a consistent peak. The diel cycle of planulation for all 3 pocilloporids and E. glabrescens suggests that the light-dark cycle may be the cue that induces planula release. The majority of planulae of these 4 species being released in the dark might be beneficial for minimizing predation effects. -

Jan 2021 London Zoo Stocklist.Pdf (596.63
ZSL London Zoo - January 2021 stocklist Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -
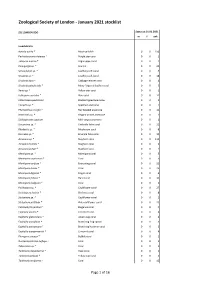
Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -

Molecular Approaches Underlying the Oogenic Cycle of the Scleractinian
www.nature.com/scientificreports OPEN Molecular approaches underlying the oogenic cycle of the scleractinian coral, Acropora tenuis Ee Suan Tan1, Ryotaro Izumi1, Yuki Takeuchi 2,3, Naoko Isomura4 & Akihiro Takemura2 ✉ This study aimed to elucidate the physiological processes of oogenesis in Acropora tenuis. Genes/ proteins related to oogenesis were investigated: Vasa, a germ cell marker, vitellogenin (VG), a major yolk protein precursor, and its receptor (LDLR). Coral branches were collected monthly from coral reefs around Sesoko Island (Okinawa, Japan) for histological observation by in situ hybridisation (ISH) of the Vasa (AtVasa) and Low Density Lipoprotein Receptor (AtLDLR) genes and immunohistochemistry (IHC) of AtVasa and AtVG. AtVasa immunoreactivity was detected in germline cells and ooplasm, whereas AtVG immunoreactivity was detected in ooplasm and putative ovarian tissues. AtVasa was localised in germline cells located in the retractor muscles of the mesentery, whereas AtLDLR was localised in the putative ovarian and mesentery tissues. AtLDLR was detected in coral tissues during the vitellogenic phase, whereas AtVG immunoreactivity was found in primary oocytes. Germline cells expressing AtVasa are present throughout the year. In conclusion, Vasa has physiological and molecular roles throughout the oogenic cycle, as it determines gonadal germline cells and ensures normal oocyte development, whereas the roles of VG and LDLR are limited to the vitellogenic stages because they act in coordination with lipoprotein transport, vitellogenin synthesis, and yolk incorporation into oocytes. Approximately 70% of scleractinian corals are hermaphroditic broadcast spawners and have both male and female gonads developing within the polyp of the same colony1. Tey engage in a multispecifc spawning event around the designated moon phase once a year2–4.