Adiabatic Process and Potential Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Thermodynamics Formulation of Economics Burin Gumjudpai A
Thermodynamics Formulation of Economics 306706052 NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 Burin Gumjudpai A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics) School of Development Economics National Institute of Development Administration 2019 Thermodynamics Formulation of Economics Burin Gumjudpai School of Development Economics Major Advisor (Associate Professor Yuthana Sethapramote, Ph.D.) The Examining Committee Approved This Thesis Submitted in Partial 306706052 Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics). Committee Chairperson NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 (Assistant Professor Pongsak Luangaram, Ph.D.) Committee (Assistant Professor Athakrit Thepmongkol, Ph.D.) Committee (Associate Professor Yuthana Sethapramote, Ph.D.) Dean (Associate Professor Amornrat Apinunmahakul, Ph.D.) ______/______/______ ABST RACT ABSTRACT Title of Thesis Thermodynamics Formulation of Economics Author Burin Gumjudpai Degree Master of Economics (Financial Economics) Year 2019 306706052 We consider a group of information-symmetric consumers with one type of commodity in an efficient market. The commodity is fixed asset and is non-disposable. We are interested in seeing if there is some connection of thermodynamics formulation NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 to microeconomics. We follow Carathéodory approach which requires empirical existence of equation of state (EoS) and coordinates before performing maximization of variables. In investigating EoS of various system for constructing the economics EoS, unexpectedly new insights of thermodynamics are discovered. With definition of truly endogenous function, criteria rules and diagrams are proposed in identifying the status of EoS for an empirical equation. -

Potential Vorticity
POTENTIAL VORTICITY Roger K. Smith March 3, 2003 Contents 1 Potential Vorticity Thinking - How might it help the fore- caster? 2 1.1Introduction............................ 2 1.2WhatisPV-thinking?...................... 4 1.3Examplesof‘PV-thinking’.................... 7 1.3.1 A thought-experiment for understanding tropical cy- clonemotion........................ 7 1.3.2 Kelvin-Helmholtz shear instability . ......... 9 1.3.3 Rossby wave propagation in a β-planechannel..... 12 1.4ThestructureofEPVintheatmosphere............ 13 1.4.1 Isentropicpotentialvorticitymaps........... 14 1.4.2 The vertical structure of upper-air PV anomalies . 18 2 A Potential Vorticity view of cyclogenesis 21 2.1PreliminaryIdeas......................... 21 2.2SurfacelayersofPV....................... 21 2.3Potentialvorticitygradientwaves................ 23 2.4 Baroclinic Instability . .................... 28 2.5 Applications to understanding cyclogenesis . ......... 30 3 Invertibility, iso-PV charts, diabatic and frictional effects. 33 3.1 Invertibility of EPV ........................ 33 3.2Iso-PVcharts........................... 33 3.3Diabaticandfrictionaleffects.................. 34 3.4Theeffectsofdiabaticheatingoncyclogenesis......... 36 3.5Thedemiseofcutofflowsandblockinganticyclones...... 36 3.6AdvantageofPVanalysisofcutofflows............. 37 3.7ThePVstructureoftropicalcyclones.............. 37 1 Chapter 1 Potential Vorticity Thinking - How might it help the forecaster? 1.1 Introduction A review paper on the applications of Potential Vorticity (PV-) concepts by Brian -

The First Law of Thermodynamics Continued Pre-Reading: §19.5 Where We Are
Lecture 7 The first law of thermodynamics continued Pre-reading: §19.5 Where we are The pressure p, volume V, and temperature T are related by an equation of state. For an ideal gas, pV = nRT = NkT For an ideal gas, the temperature T is is a direct measure of the average kinetic energy of its 3 3 molecules: KE = nRT = NkT tr 2 2 2 3kT 3RT and vrms = (v )av = = r m r M p Where we are We define the internal energy of a system: UKEPE=+∑∑ interaction Random chaotic between atoms motion & molecules For an ideal gas, f UNkT= 2 i.e. the internal energy depends only on its temperature Where we are By considering adding heat to a fixed volume of an ideal gas, we showed f f Q = Nk∆T = nR∆T 2 2 and so, from the definition of heat capacity Q = nC∆T f we have that C = R for any ideal gas. V 2 Change in internal energy: ∆U = nCV ∆T Heat capacity of an ideal gas Now consider adding heat to an ideal gas at constant pressure. By definition, Q = nCp∆T and W = p∆V = nR∆T So from ∆U = Q W − we get nCV ∆T = nCp∆T nR∆T − or Cp = CV + R It takes greater heat input to raise the temperature of a gas a given amount at constant pressure than constant volume YF §19.4 Ratio of heat capacities Look at the ratio of these heat capacities: we have f C = R V 2 and f + 2 C = C + R = R p V 2 so C p γ = > 1 CV 3 For a monatomic gas, CV = R 3 5 2 so Cp = R + R = R 2 2 C 5 R 5 and γ = p = 2 = =1.67 C 3 R 3 YF §19.4 V 2 Problem An ideal gas is enclosed in a cylinder which has a movable piston. -

Adiabatic Bulk Moduli
8.03 at ESG Supplemental Notes Adiabatic Bulk Moduli To find the speed of sound in a gas, or any property of a gas involving elasticity (see the discussion of the Helmholtz oscillator, B&B page 22, or B&B problem 1.6, or French Pages 57-59.), we need the “bulk modulus” of the fluid. This will correspond to the “spring constant” of a spring, and will give the magnitude of the restoring agency (pressure for a gas, force for a spring) in terms of the change in physical dimension (volume for a gas, length for a spring). It turns out to be more useful to use an intensive quantity for the bulk modulus of a gas, so what we want is the change in pressure per fractional change in volume, so the bulk modulus, denoted as κ (the Greek “kappa” which, when written, has a great tendency to look like k, and in fact French uses “K”), is ∆p dp κ = − , or κ = −V . ∆V/V dV The minus sign indicates that for normal fluids (not all are!), a negative change in volume results in an increase in pressure. To find the bulk modulus, we need to know something about the gas and how it behaves. For our purposes, we will need three basic principles (actually 2 1/2) which we get from thermodynamics, or the kinetic theory of gasses. You might well have encountered these in previous classes, such as chemistry. A) The ideal gas law; pV = nRT , with the standard terminology. B) The first law of thermodynamics; dU = dQ − pdV,whereUis internal energy and Q is heat. -

Session 15 Thermodynamics
Session 15 Thermodynamics Cheryl Hurkett Physics Innovations Centre for Excellence in Learning and Teaching CETL (Leicester) Department of Physics and Astronomy University of Leicester 1 Contents Welcome .................................................................................................................................. 4 Session Authors .................................................................................................................. 4 Learning Objectives .............................................................................................................. 5 The Problem ........................................................................................................................... 6 Issues .................................................................................................................................... 6 Energy, Heat and Work ........................................................................................................ 7 The First Law of thermodynamics................................................................................... 8 Work..................................................................................................................................... 9 Heat .................................................................................................................................... 11 Principal Specific heats of a gas ..................................................................................... 12 Summary .......................................................................................................................... -

1 Microphysics
ASTR 501 Stellar Physics 1 1 Microphysics • equation of state, density: ρ(P, T, X) • radiative absorption coefficient, opacity: κ(P, T, X) • rate of energy production: (P, T, X) (nuclear physics) Nuclear physics also responsible for composition changes dXj = Fˆj · Xj (1) dt burn while the total composition change also includes a mixing term: dX ∂ dX i = 4πr2ρ D i (2) dt mix ∂m dm 1.1 Equation of state For the fluid to have an EOS it must be collisional: lregion λ (3) where λ is the mean free path. P from equation of state, consider three cases: ASTR 501 Stellar Physics 2 (a) ideal gas equation of state: p = R ρT (b) barotropic equation of state: pressure p depends only on ρ, as for example • isothermal: p ∼ ρ • adiabatic: p = Kργ Plus non-ideal effects, e.g. electron degeneracy. 1.2 Ideal gas equation of state Assumption: internal energy entirely in kinetic energy according to kinetic theory: = CVT , where CV is the specific heat at constant volume. This can be seen from the first law of thermodynamics dQ = d + pdV , where V is the specific volume, d if we express d = dT dT . d Similarly, the specific heat at constant pressure Cp = dT + R where R is the gas constant. This follows from the ideal gas equation of state. [†13] It also follows that Cp − CV = R [†14], and the ratio of specific heats is defined as C γ = p . (4) CV ASTR 501 Stellar Physics 3 α CV can be related to R as CV = 2 R , where α is the number of degrees of freedom 1 per particle, since the energy per particle per degree of freedom is 2kT where k is 2+α the Boltzmann constant, and R = nk. -

Chapter 2 Approximate Thermodynamics
Chapter 2 Approximate Thermodynamics 2.1 Atmosphere Various texts (Byers 1965, Wallace and Hobbs 2006) provide elementary treatments of at- mospheric thermodynamics, while Iribarne and Godson (1981) and Emanuel (1994) present more advanced treatments. We provide only an approximate treatment which is accept- able for idealized calculations, but must be replaced by a more accurate representation if quantitative comparisons with the real world are desired. 2.1.1 Dry entropy In an atmosphere without moisture the ideal gas law for dry air is p = R T (2.1) ρ d where p is the pressure, ρ is the air density, T is the absolute temperature, and Rd = R/md, R being the universal gas constant and md the molecular weight of dry air. If moisture is present there are minor modications to this equation, which we ignore here. The dry entropy per unit mass of air is sd = Cp ln(T/TR) − Rd ln(p/pR) (2.2) where Cp is the mass (not molar) specic heat of dry air at constant pressure, TR is a constant reference temperature (say 300 K), and pR is a constant reference pressure (say 1000 hPa). Recall that the specic heats at constant pressure and volume (Cv) are related to the gas constant by Cp − Cv = Rd. (2.3) A variable related to the dry entropy is the potential temperature θ, which is dened Rd/Cp θ = TR exp(sd/Cp) = T (pR/p) . (2.4) The potential temperature is the temperature air would have if it were compressed or ex- panded (without condensation of water) in a reversible adiabatic fashion to the reference pressure pR. -
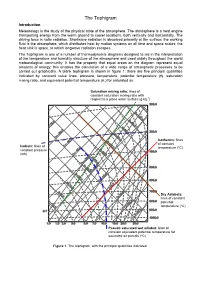
The Tephigram Introduction Meteorology Is the Study of the Physical State of the Atmosphere
The Tephigram Introduction Meteorology is the study of the physical state of the atmosphere. The atmosphere is a heat engine transporting energy from the warm ground to cooler locations, both vertically and horizontally. The driving force is solar radiation. Shortwave radiation is absorbed primarily at the surface; the working fluid is the atmosphere, which distributes heat by motion systems on all time and space scales; the heat sink is space, to which longwave radiation escapes. The Tephigram is one of a number of thermodynamic diagrams designed to aid in the interpretation of the temperature and humidity structure of the atmosphere and used widely throughout the world meteorological community. It has the property that equal areas on the diagram represent equal amounts of energy; this enables the calculation of a wide range of atmospheric processes to be carried out graphically. A blank tephigram is shown in figure 1; there are five principal quantities indicated by constant value lines: pressure, temperature, potential temperature (θ), saturation mixing ratio, and equivalent potential temperature (θe) for saturated air. Saturation mixing ratio: lines of constant saturation mixing ratio with respect to a plane water surface (g kg-1) Isotherms: lines of constant Isobars: lines of temperature (ºC) constant pressure (mb) Dry Adiabats: lines of constant potential temperature (ºC) Pseudo saturated wet adiabat: lines of constant equivalent potential temperature for saturated air parcels (ºC) Figure 1. The tephigram, with the principal quantities indicated. The principal axes of a tephigram are temperature and potential temperature; these are straight and perpendicular to each other, but rotated through about 45º anticlockwise so that lines of constant temperature run from bottom left to top right on the diagram. -

Dry Adiabatic Temperature Lapse Rate
ATMO 551a Fall 2010 Dry Adiabatic Temperature Lapse Rate As we discussed earlier in this class, a key feature of thick atmospheres (where thick means atmospheres with pressures greater than 100-200 mb) is temperature decreases with increasing altitude at higher pressures defining the troposphere of these planets. We want to understand why tropospheric temperatures systematically decrease with altitude and what the rate of decrease is. The first order explanation is the dry adiabatic lapse rate. An adiabatic process means no heat is exchanged in the process. For this to be the case, the process must be “fast” so that no heat is exchanged with the environment. So in the first law of thermodynamics, we can anticipate that we will set the dQ term equal to zero. To get at the rate at which temperature decreases with altitude in the troposphere, we need to introduce some atmospheric relation that defines a dependence on altitude. This relation is the hydrostatic relation we have discussed previously. In summary, the adiabatic lapse rate will emerge from combining the hydrostatic relation and the first law of thermodynamics with the heat transfer term, dQ, set to zero. The gravity side The hydrostatic relation is dP = −g ρ dz (1) which relates pressure to altitude. We rearrange this as dP −g dz = = α dP (2) € ρ where α = 1/ρ is known as the specific volume which is the volume per unit mass. This is the equation we will use in a moment in deriving the adiabatic lapse rate. Notice that € F dW dE g dz ≡ dΦ = g dz = g = g (3) m m m where Φ is known as the geopotential, Fg is the force of gravity and dWg is the work done by gravity. -

Introduction to Atmospheric Dynamics Chapter 2
Introduction to Atmospheric Dynamics Chapter 2 Paul A. Ullrich [email protected] Part 3: Buoyancy and Convection Vertical Structure This cooling with height is related to the dynamics of the atmosphere. The change of temperature with height is called the lapse rate. Defnition: The lapse rate is defned as the rate (for instance in K/km) at which temperature decreases with height. @T Γ ⌘@z Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Lapse Rate For a dry adiabatic, stable, hydrostatic atmosphere the potential temperature θ does not vary in the vertical direction: @✓ =0 @z In a dry adiabatic, hydrostatic atmosphere the temperature T must decrease with height. How quickly does the temperature decrease? R/cp p0 Note: Use ✓ = T p ✓ ◆ Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Lapse Rate The adiabac change in temperature with height is T @✓ @T g = + ✓ @z @z cp For dry adiabac, hydrostac atmosphere: @T g = Γd − @z cp ⌘ Defnition: The dry adiabatic lapse rate is defned as the g rate (for instance in K/km) at which the temperature of an Γd air parcel will decrease with height if raised adiabatically. ⌘ cp g 1 9.8Kkm− cp ⇡ Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Lapse Rate This profle should be very close to the adiabatic lapse rate in a dry atmosphere. Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Fundamentals Even in adiabatic motion, with no external source of heating, if a parcel moves up or down its temperature will change. • What if a parcel moves about a surface of constant pressure? • What if a parcel moves about a surface of constant height? If the atmosphere is in adiabatic balance, the temperature still changes with height. -

Heat Capacity Ratio of Gases
Heat Capacity Ratio of Gases Carson Hasselbrink [email protected] Office Hours: Mon 10-11am, Beaupre 360 1 Purpose • To determine the heat capacity ratio for a monatomic and a diatomic gas. • To understand and mathematically model reversible & irreversible adiabatic processes for ideal gases. • To practice error propagation for complex functions. 2 Key Physical Concepts • Heat capacity is the amount of heat required to raise the temperature of an object or substance one degree 풅풒 푪 = 풅푻 • Heat Capacity Ratio is the ratio of specific heats at constant pressure and constant volume 퐶푝 훾 = 퐶푣 휕퐻 휕퐸 Where 퐶 = ( ) and 퐶 = ( ) 푝 휕푇 푝 푣 휕푇 푣 • An adiabatic process occurs when no heat is exchanged between the system and the surroundings 3 Theory: Heat Capacity 휕퐸 • Heat Capacity (Const. Volume): 퐶 = ( ) /푛 푣,푚 휕푇 푣 3푅푇 3푅 – Monatomic: 퐸 = , so 퐶 = 푚표푛푎푡표푚푖푐 2 푣,푚 2 5푅푇 5푅 – Diatomic: 퐸 = , so 퐶 = 푑푖푎푡표푚푖푐 2 푣,푚 2 • Heat Capacity Ratio: 퐶푝,푚 = 퐶푣,푚 + 푅 퐶푝,푚 푅 훾 = = 1 + 퐶푣,푚 퐶푣,푚 푃 [ln 1 ] 푃2 – Reversible: 훾 = 푃 [ln 1 ] 푃3 푃 [ 1 −1] 푃2 – Irreversible: 훾 = 푃 [ 1 −1] 푃3 • Diatomic heat capacity > Monatomic Heat Capacity 4 Theory: Determination of Heat Capacity Ratio • We will subject a gas to an adiabatic expansion and then allow the gas to return to its original temperature via an isochoric process, during which time it will cool. • This expansion and warming can be modeled in two different ways. 5 Reversible Expansion (Textbook) • Assume that pressure in carboy (P1) and exterior pressure (P2) are always close enough that entire process is always in equilibrium • Since system is in equilibrium, each step must be reversible 6 Irreversible Expansion (Lab Syllabus) • Assume that pressure in carboy (P1) and exterior pressure (P2) are not close enough; there is sudden deviation in pressure; the system is not in equilibrium • Since system is not in equilibrium, the process becomes irreversible.