Ileal and Jejunal Peyer's Patches in Buffalo Calves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Anatomy of Small Intestine Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of Small Intestine Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives: At the end of the lecture, students should: List the different parts of small intestine. Describe the anatomy of duodenum, jejunum & ileum regarding: the shape, length, site of beginning & termination, peritoneal covering, arterial supply & lymphatic drainage. Differentiate between each part of duodenum regarding the length, level & relations. Differentiate between the jejunum & ileum regarding the characteristic anatomical features of each of them. Abdomen What is Mesentery? It is a double layer attach the intestine to abdominal wall. If it has mesentery it is freely moveable. L= liver, S=Spleen, SI=Small Intestine, AC=Ascending Colon, TC=Transverse Colon Abdomen The small intestines consist of two parts: 1- fixed part (no mesentery) (retroperitoneal) : duodenum 2- free (movable) part (with mesentery) :jejunum & ileum Only on the boys’ slides RELATION BETWEEN EMBRYOLOGICAL ORIGIN & ARTERIAL SUPPLY مهم :Extra Arterial supply depends on the embryological origin : Foregut Coeliac trunk Midgut superior mesenteric Hindgut Inferior mesenteric Duodenum: • Origin: foregut & midgut • Arterial supply: 1. Coeliac trunk (artery of foregut) 2. Superior mesenteric: (artery of midgut) The duodenum has 2 arterial supply because of the double origin The junction of foregut and midgut is at the second part of the duodenum Jejunum & ileum: • Origin: midgut • Arterial -

Esophageal Reconstruction Using a Pedicled Jejunum with Microvascular Augmentation
Ann Thorac Cardiovasc Surg 2011; 17: 103–109 Review Esophageal Reconstruction Using a Pedicled Jejunum with Microvascular Augmentation Takushi Yasuda, MD and Hitoshi Shiozaki, MD The pedicled colon segment is widely accepted as a substitute to the gastric tube in esopha- geal reconstruction of cases where the stomach is not available. The usefulness of reconstruc- tion with a pedicled jejunum has also been reported in recent years. In order to make a long jejunal graft, at least the second and third jejunal vessels have to be severed. However, this leads to a decrease of circulation in the pedicled jejunum. This poor circulation was primar- ily responsible for the high rates of gangrene and mortality (22.2% and 46.5%, respectively) in the beginnings of jejunal reconstruction. Advances in microsurgery have now enabled surgeons to overcome these disadvantages, as a result, both the rates of gangrene and mor- tality have decreased to almost zero since the addition of microvascular anastomosis with the jejunal vessels and the internal thoracic vessels. At present, the reconstruction using a pedi- cled jejunum is a safe operation that provides such advantages as a low incidence of intrinsic disease, more active transport of food, and a lower rate of regurgitation by peristalsis, com- pared with the reconstruction using the pedicled colon. The disadvantage of the procedure is the relatively high rate of anastomotic leakage (11.1% to 19.2%). Improvements in the surgi- cal procedures to overcome this disadvantage are, therefore, needed before it can be recom- mended without any reservations. Key words: esophageal reconstruction, jejunum, microvascular anastomosis, complication Introduction stomach is necessary. -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -

Anatomy of the Small Intestine
Anatomy of the small intestine Make sure you check this Correction File before going through the content Small intestine Fixed Free (Retro peritoneal part) (Movable part) (No mesentery) (With mesentery) Duodenum Jejunum & ileum Duodenum Shape C-shaped loop Duodenal parts Length 10 inches Length Level Beginning At pyloro-duodenal Part junction FIRST PART 2 INCHES L1 (Superior) (Transpyloric Termination At duodeno-jejunal flexure Plane) SECOND PART 3 INCHES DESCENDS Peritoneal Retroperitoneal (Descending FROM L1 TO covering L3 Divisions 4 parts THIRD PART 4 INCHES L3 (SUBCOTAL (Horizontal) PLANE) Embryologic Foregut & midgut al origin FOURTH PART 1 INCHES ASCENDS Lymphatic Celiac & superior (Ascending) FROM L3 TO drainage mesenteric L2 Arterial Celiac & superior supply mesenteric Venous Superior mesenteric& Drainage Portal veins Duodenal relations part Anterior Posterior Medial Lateral First part Liver Bile duct - - Gastroduodenal artery Portal vein Second Part Liver Transverse Right kidney Pancreas Right colic flexure Colon Small intestine Third Part Small intestine Right psoas major - - Superior Inferior vena cava mesenteric vessels Abdominal aorta Inferior mesenteric vessels Fourth Part Small intestine Left psoas major - - Openings in second part of the duodenum Opening of accessory Common opening of bile duct pancreatic duct (one inch & main pancreatic duct: higher): on summit of major duodenal on summit of minor duodenal papilla. papilla. Jejunum & ileum Shape Coiled tube Length 6 meters (20 feet) Beginning At duodeno-jejunal flexur -

Stomach, Duodenum, and Jejunum
Gut, 1970, 11, 649-658 The endocrine polypeptide cells of the human Gut: first published as 10.1136/gut.11.8.649 on 1 August 1970. Downloaded from stomach, duodenum, and jejunum A. G. E. PEARSE, I. COULLING, B. WEAVERS, AND S. FRIESEN' From the Department of Histochemistry, Royal Postgraduate Medical School, London SUMMARY Thirty specimens of stomach, duodenum, and jejunum, removed at operation, were examined by optical microscopical, cytochemical, and electron microscopical techniques. The overall distribution of four types of endocrine polypeptide cell in the stomach, and three in the intestine, was determined. The seven cell types are described by names and letters belonging to a scheme for nomenclature agreed upon at the 1969 Wiesbaden conference o* gastrointestinal hormones. The gastrin-secreting G cell was the only cell for which firm identification with a known hormone was possible. Although there was wide variation in the distribution of the various cells, from one case to another, striking differences were never- theless observable, with respect to the G cell, between antra from carcinoma and from ulcer cases. http://gut.bmj.com/ This study was undertaken with a view to estab- tive shorthand terminology. Correlation with the lishing the overall topographical distribution of terminology used by Solcia, Vassallo, and Capella the various types of endocrine polypeptide cells (1969b) and by Vassallo, Solcia, and Capella in the human stomach and upper intestine. (1969) had to be equated, if possible, with the Adequate sampling was regarded as a pre- scheme used by Forssmann, Orci, Pictet, Renold, on September 28, 2021 by guest. Protected copyright. -

What Is an Enteral Feeding Tube?
Your Feeding Tubes Feeding Your What Is an Enteral Feeding Tube? Enteral refers to within the digestive system or intestine. Enteral feeding tubes allow liquid food to enter your stomach or intestine through a tube. The soft, flexible tube enters a surgically created opening in the abdominal wall called an ostomy. An enterostomy tube in the stomach is called a gastrostomy. A tube in the small intestine is called a jejunostomy. The site on the abdomen where the tube is inserted is called a stoma. The location of the stoma depends on your specific operation and the shape of your abdomen. Most stomas: f Lie flat against your body f Are round in shape f Are red and moist (similar to the inside of your mouth) f Have no feeling Gastrostomy feeding tube Stoma tract Surgical Patient Education 112830_BODY.indd 1 8/21/15 9:25 AM 3 Who Needs an Enteral Feeding Tube? Cancer, trauma, nervous system and digestive system disorders, and congenital birth defects can cause difficulty in feeding. Some people also have difficulty swallowing, which increases the chance that they will breathe in food (aspirate). People who have difficulties feeding can benefit from a feeding tube. Your doctor will explain to you the specific reasons why you or your family member need a feeding tube. For some, a feeding tube is a new way of life, but for others, the tube is temporary and used until the problem can be treated or repaired. Low-profile feeding tube American College of Surgeons • Division of Education 112830_BODY.indd 2 8/21/15 9:25 AM 2 Your Feeding Tubes Feeding Your Understanding Your Digestive System When food enters your oral cavity (mouth), the lips and tongue move it toward the back of the throat. -

Digestive System A&P DHO 7.11 Digestive System
Digestive System A&P DHO 7.11 Digestive System AKA gastrointestinal system or GI system Function=responsible for the physical & chemical breakdown of food (digestion) so it can be taken into bloodstream & be used by body cells & tissues (absorption) Structures=divided into alimentary canal & accessory organs Alimentary Canal Long muscular tube Includes: 1. Mouth 2. Pharynx 3. Esophagus 4. Stomach 5. Small intestine 6. Large intestine 1. Mouth Mouth=buccal cavity Where food enters body, is tasted, broken down physically by teeth, lubricated & partially digested by saliva, & swallowed Teeth=structures that physically break down food by chewing & grinding in a process called mastication 1. Mouth Tongue=muscular organ, contains taste buds which allow for sweet, salty, sour, bitter, and umami (meaty or savory) sensations Tongue also aids in chewing & swallowing 1. Mouth Hard palate=bony structure, forms roof of mouth, separates mouth from nasal cavities Soft palate=behind hard palate; separates mouth from nasopharynx Uvula=cone-shaped muscular structure, hangs from middle of soft palate; prevents food from entering nasopharynx during swallowing 1. Mouth Salivary glands=3 pairs (parotid, sublingual, & submandibular); produce saliva Saliva=liquid that lubricates mouth during speech & chewing, moistens food so it can be swallowed Salivary amylase=saliva enzyme (substance that speeds up a chemical reaction) starts the chemical breakdown of carbohydrates (starches) into sugar 2. Pharynx Bolus=chewed food mixed with saliva Pharynx=throat; tube that carries air & food Air goes to trachea; food goes to esophagus When bolus is swallowed, epiglottis covers larynx which stops bolus from entering respiratory tract and makes it go into esophagus 3. -

DIGESTIVE SYSTEM -3 Emma Jakoi
Introductory Human Physiology ©copyright Emma Jakoi DIGESTIVE SYSTEM -3 Emma Jakoi. Ph.D. LEARNING OBJECTIVES 1. Explain the mechanisms of digestion and absorption of nutrients and identify where these occur within the gastrointestinal tube. 2. Explain the mechanisms of absorption of water and identify where this occurs within the gastrointestinal tube. 3. Explain the underlying mechanism for diarrhea and its causes. SMALL INTESTINE & NUTRIENT ABSORPTION Muscle contractions cause a ripple like movement that carries the food down the small intestine –like a conveyor belt. This transit is normally slow occurring over several hours. As complex food moves within the lumen of the small intestine, it is digested into small molecules. Subsequently these small molecules such as amino acids and sugars are absorbed into the body. These functions are coordinated by hormones. The small intestine is divided into three regions: duodenum, jejunum and ileum. The first, duodenum, is 10 inches long; the other two total 10 feet. The initial segment, the duodenum, receives the acidic chyme. Here the epithelium contains mucous glands and goblet cells which secrete mucus to neutralize the pH of the chyme. The duodenal epithelium cells also secrete hormones (Fig 1), cholecystokinin (CCK) and secretin, which signal the arrival of food to the pancreas, gall bladder, and stomach, respectively (Fig 1). Secretions from the pancreas and gall bladder are delivered directly to the lumen of the duodenum. Chyme G cells of stomach Duodenum CHO fats & peptides acid GLP-1 CCK Secretin Pancreas Pancreas Gall bladder Pancreas Islet Insulin enzymes bile salts HCO3- (Blood, feedforward) Figure 1. Digestive products signal the release of 2 hormones CCK and secretin from the duodenum and glucagon like peptide 1 (GLP-1) from the ileum. -

Anathomy and Phisiology of Digestive System
Danijel Borković,dr.med. Prague, 10.october 2016 Anathomy and phisiology of digestive system Digestive system: a) series of hollow organs joined in a long, twisting tube (gastointestinal tract) - food passes through them b) accessory organs – food doesnt pass through them • Other „helpers“: nerves, hormones, blood, bacteria in GI tract • Digestive system turns food and drink into nutrients (carbohydrates, protein, fats and vitamins) which body uses for energy, cell repair and growth. Anathomy and phisiology of digestive system Gastrointestinal tract: 1.Upper GI tract: a) Mouth b) Throat (pharynx) c) Esophagus d) Stomach 2. Lower GI tract: a) Small intestine b) Large intestine (with rectum) c) Anus Anathomy and phisiology of digestive system Six major functions take place in digestive system: a) Ingestion of food b) Secretion of fluids and digestyve enzymes c) Mixing and movement of food and wastes through the body d) Digestion of food into smaller pieces e) Absorption of nutrients f) Excretion of wastes Anathomy and phisiology of digestive system Mouth (oral cavity): a) Teeth b) The tongue c) Salivary glands Function: a) Chewing food: breaks it into pieces which are more easily digested. b) Saliva: mixes food to begin process of breaking it down in a form our body can absorb and use. Anathomy and phisiology of digestive system • Throat (pharynx): epiglottis as a switch between GI and RI tract • Esophagus: muscular tube extending from the pharynx to stomach. • Function: a) Deliveres food to stomach with series of contractions – peristalsis. -

Gastrointestinal Tract 4: Anatomy and Role of the Jejunum and Ileum
Copyright EMAP Publishing 2019 This article is not for distribution except for journal club use Clinical Practice Keywords Villi/Microvilli/Absorption/ Segmentation/Vitamin B complex Systems of life This article has been GI tract double-blind peer reviewed In this article... ● Role of the jejunum and ileum in chemical digestion and absorption of nutrients ● Nutrient absorption from the small intestine to the bloodstream via the villi ● Processes of segmentation and peristalsis Gastrointestinal tract 4: anatomy and role of the jejunum and ileum Key points Authors Yamni Nigam is professor in biomedical science; John Knight is associate The small intestine professor in biomedical science; Nikki Williams is associate professor in respiratory comprises the physiology; all at the College of Human and Health Sciences, Swansea University. duodenum, jejunum and ileum Abstract After its passage through the duodenum, where most chemical digestion takes place, chyme passes through the jejunum and ileum. Their main role is to ensure The jejunum and that the various molecules resulting from chemical digestion pass through the gut ileum finish chemical wall into the blood or lymph. This process of nutrient absorption is helped by the digestion and presence of folds and projections that hugely increase the surface area of the gut absorb most of wall, and regular contractions of the rings of smooth muscle that move intestinal the nutrients contents back and forth. This article, the fourth in a six-part series exploring the gastrointestinal tract, describes the anatomy and functions of the jejunum and ileum. Folds and projections in the Citation Nigam Y et al (2019) Gastrointestinal tract 4: anatomy and role of the small intestine’s wall jejunum and ileum. -
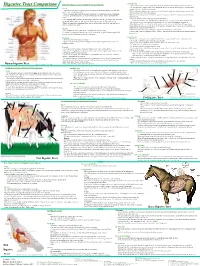
Digestive Tract Comparison • the Small Intestine Is a Tube Roughly Twenty Feet Long Deided Into the Duodenum, Jejunum and Ileum
• Small Intestine Human/Dog Digestive system or Simple Monogastric Digestion Digestive Tract Comparison • The small intestine is a tube roughly twenty feet long deided into the duodenum, jejunum and ileum. • The first part of the small intestine is the duodenum, the site of most chemical digestive reactions and is Mouth smoother than the rest of the small intestine • A specialized region of the digestive tract designed to break up large particles of food into • Bile, bicarbonate and pancreatic enzymes are secreted into the duodenum to breakdown nutrients in the smaller, more manageable particles chyme so that they can be readily absorbed. • Saliva is added to moisten food and begin carbohydrate breakdown by amylase in humans. •Bicarbonate from the pancreas neutralizes corrosive stomach acid from 3.5 in the stomach to 8.5 in the • There are four main types of teeth in the human or dog: incisors, canines, premolars and small intestine. molars. •Pancreatic enzymes include lipases, peptidases and amylases. •One reason dog and cat canines are much larger than ours is that they need to be able to rip and •Lipases break down fats. Peptidases break down proteins. Amylases break down carbohydrates. tear through tough raw meat. Humans have evolved to eat easier to chew, cooked meat. • Bile from the liver is stored in the gall bladder and secreted into the duodenum to emulsify fat. • While chewing, food is transformed into what is called a bolus, a food ball, and then forced •The jejunum and ileum are next in the small intestine and are covered in villi, finger-like projections. -

Small Intestines of the Horse
SMALL INTESTINES OF THE HORSE Duodenum: The cranial part of the duodenum is in contact with the visceral surface of the liver. It forms the sigmoid flexure and a dilatation (ampulla). The first curve of the sigmoid flexure is convex dorsally and the second curve is convex ventrally. The second curve of the sigmoid flexure is the cranial flexure where the body of the pancreas is attached. The major pancreatic duct and the bile duct open in this area at the major duodenal papilla inside the hepatopancreatic ampulla . The accessory pancreatic duct opens at the minor duodenal papilla opposite to the major duodenal papilla. The descending duodenum runs caudally between the visceral surface of the liver and the right dorsal colon. At the caudal pole of the right kidney it turns medially around the base of the cecum and the root of the mesentery to form the caudal flexure. The short ascending duodenum runs cranially and to the left. It is continued by the jejunum following the duodenojejunal flexure . Jejunum: Lies chiefly in the left dorsal part of the abdomen together with the small colon. It is attached to the dorsal abdominal wall by the long mesojejunum that allows great mobility of the bowel . This mobility may result in intestinal colic, due to volvulus, intussusception, or incarceration of the duodenal loops in the epiploic foramen or the vaginal ring. Ileum: Passes dorsally toward the lesser curvature of the base of the cecum, where it is partly telescoped into the the cecum so that the ileal orifice is surrounded by a fold of mucous membrane.