Diagnosis and Differential Diagnosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Segmental Overvekst Og Vaskulærmalformasjoner V02
2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Avdeling for medisinsk genetikk Segmental overvekst og vaskulærmalformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* ACVRL1 175 NM_000020.3 2-10 Telangiectasia, hereditary hemorrhagic, type 2 OMIM ADAMTS3 219 NM_014243.3 1-22 Hennekam lymphangiectasia- lymphedema syndrome 3 OMIM AKT1 391 NM_005163.2 2-14 Cowden syndrome 6 OMIM Proteus syndrome, somatic OMIM AKT2 392 NM_001626.6 2-14 Diabetes mellitus, type II OMIM Hypoinsulinemic hypoglycemia with hemihypertrophy OMIM AKT3 393 NM_005465.7 2-14 Megalencephaly-polymicrogyria- polydactyly-hydrocephalus syndrome 2 OMIM file:///data/SegOv_v02-web.html 1/7 2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Ekson Gen Gen affisert (HGNC (HGNC -

Central Nervous System Tumors General ~1% of Tumors in Adults, but ~25% of Malignancies in Children (Only 2Nd to Leukemia)
Last updated: 3/4/2021 Prepared by Kurt Schaberg Central Nervous System Tumors General ~1% of tumors in adults, but ~25% of malignancies in children (only 2nd to leukemia). Significant increase in incidence in primary brain tumors in elderly. Metastases to the brain far outnumber primary CNS tumors→ multiple cerebral tumors. One can develop a very good DDX by just location, age, and imaging. Differential Diagnosis by clinical information: Location Pediatric/Young Adult Older Adult Cerebral/ Ganglioglioma, DNET, PXA, Glioblastoma Multiforme (GBM) Supratentorial Ependymoma, AT/RT Infiltrating Astrocytoma (grades II-III), CNS Embryonal Neoplasms Oligodendroglioma, Metastases, Lymphoma, Infection Cerebellar/ PA, Medulloblastoma, Ependymoma, Metastases, Hemangioblastoma, Infratentorial/ Choroid plexus papilloma, AT/RT Choroid plexus papilloma, Subependymoma Fourth ventricle Brainstem PA, DMG Astrocytoma, Glioblastoma, DMG, Metastases Spinal cord Ependymoma, PA, DMG, MPE, Drop Ependymoma, Astrocytoma, DMG, MPE (filum), (intramedullary) metastases Paraganglioma (filum), Spinal cord Meningioma, Schwannoma, Schwannoma, Meningioma, (extramedullary) Metastases, Melanocytoma/melanoma Melanocytoma/melanoma, MPNST Spinal cord Bone tumor, Meningioma, Abscess, Herniated disk, Lymphoma, Abscess, (extradural) Vascular malformation, Metastases, Extra-axial/Dural/ Leukemia/lymphoma, Ewing Sarcoma, Meningioma, SFT, Metastases, Lymphoma, Leptomeningeal Rhabdomyosarcoma, Disseminated medulloblastoma, DLGNT, Sellar/infundibular Pituitary adenoma, Pituitary adenoma, -

Phenotypic and Genotypic Characterisation of Noonan-Like
1of5 ELECTRONIC LETTER J Med Genet: first published as 10.1136/jmg.2004.024091 on 2 February 2005. Downloaded from Phenotypic and genotypic characterisation of Noonan-like/ multiple giant cell lesion syndrome J S Lee, M Tartaglia, B D Gelb, K Fridrich, S Sachs, C A Stratakis, M Muenke, P G Robey, M T Collins, A Slavotinek ............................................................................................................................... J Med Genet 2005;42:e11 (http://www.jmedgenet.com/cgi/content/full/42/2/e11). doi: 10.1136/jmg.2004.024091 oonan-like/multiple giant cell lesion syndrome (NL/ MGCLS; OMIM 163955) is a rare condition1–3 with Key points Nphenotypic overlap with Noonan’s syndrome (OMIM 163950) and cherubism (OMIM 118400) (table 1). N Noonan-like/multiple giant cell lesion syndrome (NL/ Recently, missense mutations in the PTPN11 gene on MGCLS) has clinical similarities with Noonan’s syn- chromosome 12q24.1 have been identified as the cause of drome and cherubism. It is unclear whether it is a Noonan’s syndrome in 45% of familial and sporadic cases,45 distinct entity or a variant of Noonan’s syndrome or indicating genetic heterogeneity within the syndrome. In the cherubism. 5 study by Tartaglia et al, there was a family in which three N Three unrelated patients with NL/MGCLS were char- members had features of Noonan’s syndrome; two of these acterised, two of whom were found to have mutations had incidental mandibular giant cell lesions.3 All three in the PTPN11 gene, the mutation found in 45% of members were found to have a PTPN11 mutation known to patients with Noonan’s syndrome. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

Revision of Diagnostic Criteria for Neurofibromatosis
Revision of Diagnostic Criteria for Neurofibromatosis Gareth Evans, MD, St. Mary’s Hospital/University of Manchester, UK Susan Huson, MD, Consultant Geneticist, UK Eric Legius, MD, PhD, University of Leuven, Belgium Ludwine Messiaen, PhD, University of AlaBama, Birmingham, USA Scott Plotkin, MD, PhD, Massachusetts General Hospital, USA Pierre Wolkenstein, MD, PhD, Hôpital Henri-Mondor, France 2019 NF Conference San Francisco, CA September 21, 2019 Revision of the Diagnostic Criteria of the Neurofibromatoses 92 medical specialists 20 countries 5 continents We want your feedback! Send email to: [email protected] Participants in revision process: Leadership team Clinical Epidemiology Health Services Research: Ophthalmology: • Gareth Evans • Nilton Rezende • Vanessa Merker • Robert Avery • Sue Huson • Catherine Cassiman • Eric Legius Clinical Genetics: Internal Medicine: Pathology: • Ludwine Messiaen • Dorothy Halliday • Luiz Oswaldo Carneiro • Karin Cunha • Scott Plotkin • Maurizio Clementi Rodrigues • Anat Stemmer-Rachamimov • Pierre Wolkenstein • Arvid Heiberg • Patrice Pancza • Joanne Ngeow Scientists: Pediatrics: • Shay Ben-Schachar • Miriam Smith • Outside Experts: • Bruce Korf • Marco Giovannini Rianne Oostenbrink • • Monique Anten • Mimi Berman • Eduard Serra Robert Listernick • Jaan Toelen • Betty Schorry • Juha Peltonen Pediatric Hematology and • Arthur Aylsworth • Gareth Evans Oncology: • Meredith Wilson • Ignacio Blanco Molecular Genetics: • Michael Fisher • Helen Hanson • Dusica Babovic-Vuksanovic •Laura Papi • Christopher -

Cerebellar Anaplastic Astrocytoma in Adult Patients: 15 Consecutive Cases from a Single Institution and Literature Review
medRxiv preprint doi: https://doi.org/10.1101/2020.09.09.20188938; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Cerebellar anaplastic astrocytoma in adult patients: 15 consecutive cases from a single institution and literature review Artem Belyaev1, Dmitry Usachev1, Marina Ryzhova1, Gleb Gulida1, Vasilisa Skvortsova2,3, Igor Pronin1, Grigory Kobiakov1. 1 Burdenko Neurosurgery Center, 4th Tverskaya-Yamskaya, 16, Moscow, 125047, Russia 2 Wellcome Trust Centre for Neuroimaging, University College London, London, United Kingdom; 3 Max Planck University College London Centre for Computational Psychiatry and Ageing Research, London, United Kingdom; Abstract: Adult cerebellar anaplastic astrocytomas (cAA) are rare entities and their clinical and genetic appearances are still ill defined. Previously, malignant gliomas of the cerebellum were combined and reviewed together (cAA and cerebellar glioblastomas (cGB), that could have possibly affected overall results. We present characteristics of 15 adult patients with cAA and compared them to a series of 45 patients with a supratentorial AA (sAA). The mean age at cAA diagnosis was 39.3 years (range 19-72). A history of neurofibromatosis type I was noted in 1 patient (6.7%). An IDH-1 mutation was identified in 6/15 cases and a methylated MGMT promoter in 5/15 cases. Patients in study and control groups were matched in age, sex and IDH- 1 mutation status. Patients in a study group tended to have a more frequent multifocal presentation at diagnosis (13% vs. -

Cardiomyopathy Precision Panel Overview Indications
Cardiomyopathy Precision Panel Overview Cardiomyopathies are a group of conditions with a strong genetic background that structurally hinder the heart to pump out blood to the rest of the body due to weakness in the heart muscles. These diseases affect individuals of all ages and can lead to heart failure and sudden cardiac death. If there is a family history of cardiomyopathy it is strongly recommended to undergo genetic testing to be aware of the family risk, personal risk, and treatment options. Most types of cardiomyopathies are inherited in a dominant manner, which means that one altered copy of the gene is enough for the disease to present in an individual. The symptoms of cardiomyopathy are variable, and these diseases can present in different ways. There are 5 types of cardiomyopathies, the most common being hypertrophic cardiomyopathy: 1. Hypertrophic cardiomyopathy (HCM) 2. Dilated cardiomyopathy (DCM) 3. Restrictive cardiomyopathy (RCM) 4. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) 5. Isolated Left Ventricular Non-Compaction Cardiomyopathy (LVNC). The Igenomix Cardiomyopathy Precision Panel serves as a diagnostic and tool ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes. Indications The Igenomix Cardiomyopathy Precision Panel is indicated in those cases where there is a clinical suspicion of cardiomyopathy with or without the following manifestations: - Shortness of breath - Fatigue - Arrythmia (abnormal heart rhythm) - Family history of arrhythmia - Abnormal scans - Ventricular tachycardia - Ventricular fibrillation - Chest Pain - Dizziness - Sudden cardiac death in the family 1 Clinical Utility The clinical utility of this panel is: - The genetic and molecular diagnosis for an accurate clinical diagnosis of a patient with personal or family history of cardiomyopathy, channelopathy or sudden cardiac death. -
A Case of Intramedullary Spinal Cord Astrocytoma Associated with Neurofibromatosis Type 1
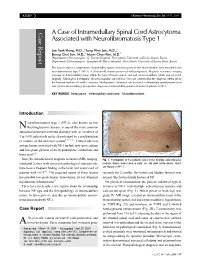
KISEP J Korean Neurosurg Soc 36 : 69-71, 2004 Case Report A Case of Intramedullary Spinal Cord Astrocytoma Associated with Neurofibromatosis Type 1 Jae Taek Hong, M.D.,1 Sang Won Lee, M.D.,1 Byung Chul Son, M.D.,1 Moon Chan Kim, M.D.2 Department of Neurosurgery,1 St. Vincent Hospital, The Catholic University of Korea, Suwon, Korea Department of Neurosurgery,2 Kangnam St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea The authors report a symptomatic intramedullary spinal cord astrocytoma in the thoracolumbar area associated with neurofibromatosis type 1 (NF-1). A 38-year-old woman presented with paraparesis. Magnetic resonance imaging revealed an intramedullary lesion within the lower thoracic spinal cord and conus medullaris, which was removed surgically. Pathological investigation showed anaplastic astrocytoma. This case confirms that the diagnosis criteria set by the National Institute of Health Consensus Development Conference can be useful to differentiate ependymoma from astrocytoma when making a preoperative diagnosis of intramedullary spinal cord tumor in patients of NF-1. KEY WORDS : Astrocytoma·Intramedullary cord tumor·Neurofibromatosis. Introduction eurofibromatosis type 1 (NF-1), also known as von N Recklinghausen's disease, is one of the most common autosomal dominant inherited disorders with an incidence of 1 in 3,000 individuals and is characterized by a predisposition to tumors of the nervous system5,6,12,16). Central nervous system lesions associated with NF-1 include optic nerve glioma and low-grade gliomas of the hypothalamus, cerebellum and brain stem6,10). Since the introduction of magnetic resonance(MR) imaging, Fig. 1. Photograph of the patient's back shows multiple subcutaneous incidental lesions with uncertain pathological characteristic nodules (black arrow) and a cafe-au-lait spot (white arrow), which have been a frequent finding in the brain and spinal cord of are typical of NF-1. -

2018 Abstract Book
CONTENTS Table of Contents INFORMATION Continuing Medical Education .................................................................................................5 Guidelines for Speakers ..........................................................................................................6 Guidelines for Poster Presentations .........................................................................................8 SPEAKER ABSTRACTS Abstracts ...............................................................................................................................9 POSTER ABSTRACTS Basic Research (Location – Room 101) ...............................................................................63 Clinical (Location – Room 8) ..............................................................................................141 2018 Joint Global Neurofibromatosis Conference · Paris, France · November 2-6, 2018 | 3 4 | 2018 Joint Global Neurofibromatosis Conference · Paris, France · November 2-6, 2018 EACCME European Accreditation Council for Continuing Medical Education 2018 Joint Global Neurofibromatosis Conference Paris, France, 02/11/2018–06/11/2018 has been accredited by the European Accreditation Council for Continuing Medical Education (EACCME®) for a maximum of 27 European CME credits (ECMEC®s). Each medical specialist should claim only those credits that he/she actually spent in the educational activity. The EACCME® is an institution of the European Union of Medical Specialists (UEMS), www.uems.net. Through an agreement between -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Whole Exome Sequencing Gene Package Intellectual Disability, Version 9.1, 31-1-2020
Whole Exome Sequencing Gene package Intellectual disability, version 9.1, 31-1-2020 Technical information DNA was enriched using Agilent SureSelect DNA + SureSelect OneSeq 300kb CNV Backbone + Human All Exon V7 capture and paired-end sequenced on the Illumina platform (outsourced). The aim is to obtain 10 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate and non-unique reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA-MEM algorithm (reference: http://bio-bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A2ML1 {Otitis media, susceptibility to}, 166760 610627 66 100 100 96 AARS1 Charcot-Marie-Tooth disease, axonal, type 2N, 613287 601065 63 100 97 90 Epileptic encephalopathy, early infantile, 29, 616339 AASS Hyperlysinemia, -

Astrocytoma: a Hormone-Sensitive Tumor?
International Journal of Molecular Sciences Review Astrocytoma: A Hormone-Sensitive Tumor? Alex Hirtz 1, Fabien Rech 1,2,Hélène Dubois-Pot-Schneider 1 and Hélène Dumond 1,* 1 Université de Lorraine, CNRS, CRAN, F-54000 Nancy, France; [email protected] (A.H.); [email protected] (F.R.); [email protected] (H.D.-P.-S.) 2 Université de Lorraine, CHRU-Nancy, Service de Neurochirurgie, F-54000 Nancy, France * Correspondence: [email protected]; Tel.: +33-372746115 Received: 29 October 2020; Accepted: 27 November 2020; Published: 30 November 2020 Abstract: Astrocytomas and, in particular, their most severe form, glioblastoma, are the most aggressive primary brain tumors and those with the poorest vital prognosis. Standard treatment only slightly improves patient survival. Therefore, new therapies are needed. Very few risk factors have been clearly identified but many epidemiological studies have reported a higher incidence in men than women with a sex ratio of 1:4. Based on these observations, it has been proposed that the neurosteroids and especially the estrogens found in higher concentrations in women’s brains could, in part, explain this difference. Estrogens can bind to nuclear or membrane receptors and potentially stimulate many different interconnected signaling pathways. The study of these receptors is even more complex since many isoforms are produced from each estrogen receptor encoding gene through alternative promoter usage or splicing, with each of them potentially having a specific role in the cell. The purpose of this review is to discuss recent data supporting the involvement of steroids during gliomagenesis and to focus on the potential neuroprotective role as well as the mechanisms of action of estrogens in gliomas.