Functions of Maize Genes Encoding Pyruvate Phosphate Dikinase in Developing Endosperm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

METACYC ID Description A0AR23 GO:0004842 (Ubiquitin-Protein Ligase
Electronic Supplementary Material (ESI) for Integrative Biology This journal is © The Royal Society of Chemistry 2012 Heat Stress Responsive Zostera marina Genes, Southern Population (α=0. -

Characterization and Phylogeny of the Pfp Gene of Amycolatopsis Methanolica Encoding
JOURNAL OF BACTERIOLOGY, Jan. 1996, p. 149–155 Vol. 178, No. 1 0021-9193/96/$04.0010 Copyright q 1996, American Society for Microbiology Characterization and Phylogeny of the pfp Gene of Amycolatopsis methanolica Encoding PPi-Dependent Phosphofructokinase ALEXANDRA M. C. R. ALVES, WIM G. MEIJER, JAN W. VRIJBLOED, AND LUBBERT DIJKHUIZEN* Department of Microbiology, Groningen Biomolecular Sciences and Biotechnology Institute (GBB), University of Groningen, 9751 NN Haren, The Netherlands Received 28 July 1995/Accepted 2 November 1995 The actinomycete Amycolatopsis methanolica employs a PPi-dependent phosphofructokinase (PPi-PFK) (EC 2.7.1.90) with biochemical characteristics similar to those of both ATP- and PPi-dependent enzymes during growth on glucose. A 2.3-kb PvuII fragment hybridizing to two oligonucleotides based on the amino-terminal amino acid sequence of PPi-PFK was isolated from a genomic library of A. methanolica. Nucleotide sequence analysis of this fragment revealed the presence of an open reading frame encoding a protein of 340 amino acids with a high degree of similarity to PFK proteins. Heterologous expression of this open reading frame in Escherichia coli gave rise to a unique 45-kDa protein displaying a high level of PPi-PFK activity. The open reading frame was therefore designated pfp, encoding the PPi-PFK of A. methanolica. Upstream and transcribed divergently from pfp, a partial open reading frame (aroA) similar to 3-deoxy-D-arabino-heptulosonate-7- phosphate synthase-encoding genes was identified. The partial open reading frame (chiA) downstream from pfp was similar to chitinase genes from Streptomyces species. A phylogenetic analysis of the ATP- and PPi- dependent proteins showed that PPi-PFK enzymes are monophyletic, suggesting that the two types of PFK evolved from a common ancestor. -

In the Field of Clinical Examination, the Measurement of Creatine
J. Clin. Biochem. Nutr., 3, 17-25, 1987 Bacterial Glucokinase as an Enzymic Reagent of Good Stability for Measurement of Creatine Kinase Activity Hitoshi KONDO, * Takanari SHIRAISHI, Masao KAGEYAMA, Kazuhiko NAGATA, and Kosuke TOMITA Research and Development Center, UNITIKA Ltd., Uji 611, Japan (Received January 10, 1987) Summary An enzymic reagent, that has long-term stability even in the liquid state, was successfully employed for the measurement of serum creatine kinase (CK, EC 2.7.3.2) activity. The enzyme used was the thermostable glucokinase (GlcK, EC 2.7.1.2) obtained from the thermo- phile Bacillus stearothermophilus. The reagent was found to be stable in solution for about one month at 6•Ž and for about one week at 30•Ž. This substitution of glucokinase for the hexokinase of the most commonly used hexokinase-glucose-6-phosphate dehydrogenase (HK-G6PDH) method results in a remarkable improvement of the method. The CK activity measured by the GlcK-G6PDH method was linear up to about 2,000 U/ liter at 37•Ž. The GlcK-G6PDH method was found to give a satisfactory precision and reproducibility (coefficient of variation less than 2.17%). Over a wide range of CK activity, an excellent agreement was obtained between the GlcK-G6PDH and the HK-G6PDH methods. Furthermore several coexistents and anticoagulants were found to have little effect on the measured value of CK activity by the GlcK-G6PDH method. Key Words: creatine kinase activity, glucokinase, improved stability of reagent, creatine kinase determination, thermostable enzyme In the field of clinical examination, the measurement of creatine kinase (CK, ATP : creatine phosphotransferase, EC 2.7.3.2) activity in serum is one of the important examinations usually employed for diagnosis of cardiac diseases such as myocardial infarction or muscular diseases such as progressive muscular dystrophy. -

Hereditary Galactokinase Deficiency J
Arch Dis Child: first published as 10.1136/adc.46.248.465 on 1 August 1971. Downloaded from Alrchives of Disease in Childhood, 1971, 46, 465. Hereditary Galactokinase Deficiency J. G. H. COOK, N. A. DON, and TREVOR P. MANN From the Royal Alexandra Hospital for Sick Children, Brighton, Sussex Cook, J. G. H., Don, N. A., and Mann, T. P. (1971). Archives of Disease in Childhood, 46, 465. Hereditary galactokinase deficiency. A baby with galactokinase deficiency, a recessive inborn error of galactose metabolism, is des- cribed. The case is exceptional in that there was no evidence of gypsy blood in the family concerned. The investigation of neonatal hyperbilirubinaemia led to the discovery of galactosuria. As noted by others, the paucity of presenting features makes early diagnosis difficult, and detection by biochemical screening seems desirable. Cataract formation, of early onset, appears to be the only severe persisting complication and may be due to the biosynthesis and accumulation of galactitol in the lens. Ophthalmic surgeons need to be aware of this enzyme defect, because with early diagnosis and dietary treatment these lens changes should be reversible. Galactokinase catalyses the conversion of galac- and galactose diabetes had been made in this tose to galactose-l-phosphate, the first of three patient (Fanconi, 1933). In adulthood he was steps in the pathway by which galactose is converted found to have glycosuria as well as galactosuria, and copyright. to glucose (Fig.). an unexpectedly high level of urinary galactitol was detected. He was of average intelligence, and his handicaps, apart from poor vision, appeared to be (1) Galactose Gackinase Galactose-I-phosphate due to neurofibromatosis. -

Isozymes of Pyruvate Kinase in Liver and Hepatomas of the Rat1
[CANCER RESEARCH 34, 1439-1446, June 1974] Isozymes of Pyruvate Kinase in Liver and Hepatomas of the Rat1 Francis A. Farina,2 Jennie B. Shatton, Harold P. Morris, and Sidney Weinhouse The Fels Research Institute and the Department of Biochemistry, Temple University School oj Medicine, Philadelphia. Pennsylvania IV140 (F. A. F., J. B. S.. S. W.\, and the Department of Biochemistry. Howard University School of Medicine. Washington. D. C. 20001 [H. P. M .\ SUMMARY 23, 32, 41, 57). These alterations involve the replacement of those isozymes that are under dietary and hormonal control Pyruvate kinase (PK) (EC 2.7.1.40) isozymes were assayed by the host, and that have important metabolic functions in in normal rat liver and a series of transplantable rat the adult differentiated liver by other isozymes which are hepatomas ranging widely in growth rate and degree of normally either low in, or absent from, the adult tissue. As differentiation, with the use of gradient elution by chloride part of an ongoing study of this phenomenon, we have ion from columns of DEAE-cellulose. In agreement with examined the alteration of PK3 (EC 2.7.1.40) isozymes in other studies, three noninterconvertible forms were found in the Morris hepatomas (30. 31), a series of chemically rat tissues: isozyme I, the major form in adult rat liver: induced, transplantable rat hepatomas ranging widely in isozyme II, the sole form in heart and skeletal muscle: and growth rate and degree of differentiation. This enzyme isozyme III, the sole form in poorly differentiated hepato occupies a key position in the metabolism of cells and, as we mas, the major form in normal kidney and lung, and the pointed out previously (27, 28. -

Labeled in Thecourse of Glycolysis, Since Phosphoglycerate Kinase
THE STATE OF MAGNESIUM IN CELLS AS ESTIMATED FROM THE ADENYLATE KINASE EQUILIBRIUM* BY TRWIN A. RoSE THE INSTITUTE FOR CANCER RESEARCH, PHILADELPHIA Communicated by Thomas F. Anderson, August 30, 1968 Magnesium functions in many enzymatic reactions as a cofactor and in com- plex with nucleotides acting as substrates. Numerous examples of a possible regulatory role of Mg can be cited from studies with isolated enzymes,'- and it is known that Mg affects the structural integrity of macromolecules such as trans- fer RNA" and functional elements such as ribosomes.'0 The major problem in translating this information on isolated preparations to the functioning cell is the difficulty in determining the distribution of Mg and the nucleotides among the free and complexed forms that function in the region of the cell for which this information is desired. Nanningall based an attempt to calculate the free Mg2+ and Ca2+ ion concentrations of frog muscle on the total content of these metals and of the principal known ligands (adenosine 5'-triphosphate (ATP), creatine-P, and myosin) and the dissociation constants of the complexes. However, this method suffers from the necessity of evaluating the contribution of all ligands as well as from the assumption that all the known ligands are contributing their full complexing capacity. During studies concerned with the control of glycolysis in red cells and the control of the phosphoglycerate kinase step in particular, it became important to determine the fractions of the cell's ATP and adenosine 5'-diphosphate (ADP) that were present as Mg complexes. Just as the problem of determining the distribution of protonated and dissociated forms of an acid can be solved from a knowledge of pH and pKa of the acid, so it would be possible to determine the liganded and free forms of all rapidly established Mg complexes from a knowledge of Mg2+ ion concentration and the appropriate dissociation constants. -

Induction of Uridyl Transferase Mrna-And Dependency on GAL4 Gene Function (In Vitro Translation/Immunoprecipitation/GAL Gene Cluster/Positive Regulation) JAMES E
Proc. Nati. Acad. Sci. USA Vol. 75, No. 6, pp. 2878-2882, June 1978 Genetics Regulation of the galactose pathway in Saccharomyces cerevisiae: Induction of uridyl transferase mRNA-and dependency on GAL4 gene function (in vitro translation/immunoprecipitation/GAL gene cluster/positive regulation) JAMES E. HOPPER*, JAMES R. BROACHt, AND LUCY B. ROWE* * Rosenstiel Basic Medical Sciences Research Center, Brandeis University, Waltham, Massachusetts 02154; and t Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724 Communicated by Norman H. Giles, April 10,1978 ABSTRACT In Saccharomyces cerevisiae, utilization of Genetic control of the inducible galactose pathway enzymes galactose requires four inducible enzyme activities. Three of involves the four structural genes GALI, GAL10, GAL7, and these activities (galactose-l-phosphate uridyl transferase, EC genes, GAL4, GAL81 (c), GAL80 2.7.7.10; uridine diphosphogalactose 4-epimerase, EC 5.1.3.2; GAL2 and four regulatory and galactokinase, EC 2.7.1.6) are specified by three tightly (i), and GALS.* Mutations in GALl, GAL10, GAL7, and GAL2 linked genes (GAL7, GALlO, and GALI, respectively) on chro- affect the individual appearance of galactokinase, epimerase, mosome II, whereas the fourth, galactose transport, is specified transferase, and galactose transport activities, respectively (6). by a gene (GALS) located on chromosome XIL Although classic Mutations defining the GALl, GAL10, and GAL7 genes have genetic analysis has revealed both positive and negative regu- invariably been recessive, and they map in three tightly linked latory genes that coordinately affect the appearance of ail four complementation groups near the centromere of chromosome enzyme activities, neither the basic events leading to the ap- pearance of enzyme activities nor the roles of the regulatory II (6, 9, 10). -

Pyruvate Kinase: Function, Regulation and Role in Cancer
Pyruvate kinase: Function, regulation and role in cancer The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Israelsen, William J., and Matthew G. Vander Heiden. “Pyruvate Kinase: Function, Regulation and Role in Cancer.” Seminars in Cell & Developmental Biology 43 (2015): 43–51. As Published http://dx.doi.org/10.1016/j.semcdb.2015.08.004 Publisher Elsevier Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/105833 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ HHS Public Access Author manuscript Author Manuscript Author ManuscriptSemin Cell Author Manuscript Dev Biol. Author Author Manuscript manuscript; available in PMC 2016 August 13. Published in final edited form as: Semin Cell Dev Biol. 2015 July ; 43: 43–51. doi:10.1016/j.semcdb.2015.08.004. Pyruvate kinase: function, regulation and role in cancer William J. Israelsena,1,* and Matthew G. Vander Heidena,b,* aKoch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA bDepartment of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA 02115, USA Abstract Pyruvate kinase is an enzyme that catalyzes the conversion of phosphoenolpyruvate and ADP to pyruvate and ATP in glycolysis and plays a role in regulating cell metabolism. There are four mammalian pyruvate kinase isoforms with unique tissue expression patterns and regulatory properties. The M2 isoform of pyruvate kinase (PKM2) supports anabolic metabolism and is expressed both in cancer and normal tissue. The enzymatic activity of PKM2 is allosterically regulated by both intracellular signaling pathways and metabolites; PKM2 thus integrates signaling and metabolic inputs to modulate glucose metabolism according to the needs of the cell. -
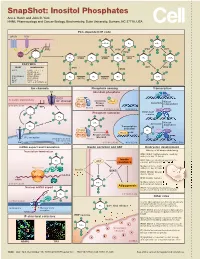
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

The Characterization of Human Adenylate Kinases 7 and 8
The characterization of human adenylate kinases 7 and 8 demonstrates differences in kinetic parameters and structural organization among the family of adenylate kinase isoenzymes Christakis Panayiotou, Nicola Solaroli, Yunjian Xu, Magnus Johansson, Anna Karlsson To cite this version: Christakis Panayiotou, Nicola Solaroli, Yunjian Xu, Magnus Johansson, Anna Karlsson. The char- acterization of human adenylate kinases 7 and 8 demonstrates differences in kinetic parameters and structural organization among the family of adenylate kinase isoenzymes. Biochemical Journal, Port- land Press, 2011, 433 (3), pp.527-534. 10.1042/BJ20101443. hal-00558097 HAL Id: hal-00558097 https://hal.archives-ouvertes.fr/hal-00558097 Submitted on 21 Jan 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Biochemical Journal Immediate Publication. Published on 16 Nov 2010 as manuscript BJ20101443 The characterization of human adenylate kinases 7 and 8 demonstrates differences in kinetic parameters and structural organization among the family of adenylate kinase isoenzymes -

Role of Glucokinase and Glucose-6 Phosphatase in the Nutritional Regulation of Endogenous Glucose Production G Mithieux
Role of glucokinase and glucose-6 phosphatase in the nutritional regulation of endogenous glucose production G Mithieux To cite this version: G Mithieux. Role of glucokinase and glucose-6 phosphatase in the nutritional regulation of endogenous glucose production. Reproduction Nutrition Development, EDP Sciences, 1996, 36 (4), pp.357-362. hal-00899845 HAL Id: hal-00899845 https://hal.archives-ouvertes.fr/hal-00899845 Submitted on 1 Jan 1996 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Review Role of glucokinase and glucose-6 phosphatase in the nutritional regulation of endogenous glucose production G Mithieux Unité 197 de l’Inserm, faculté de médecine René-Laënnec, rue Guillaume-Paradin, 69372 Lyon cedex 08, France (Received 29 November 1995; accepted 6 May 1996) Summary ― Two specific enzymes, glucokinase (GK) and glucose-6 phosphatase (Gic6Pase) enable the liver to play a crucial role in glucose homeostasis. The activity of Glc6Pase, which enables the liver to produce glucose, is increased during short-term fasting, in agreement with the enhancement of liver gluconeogenesis. During long-term fasting, Glc6Pase activity is increased in the kidney, which con- tributes significantly to the glucose supply at that time. -

Pyruvate-Phosphate Dikinase of Oxymonads and Parabasalia and the Evolution of Pyrophosphate-Dependent Glycolysis in Anaerobic Eukaryotes† Claudio H
EUKARYOTIC CELL, Jan. 2006, p. 148–154 Vol. 5, No. 1 1535-9778/06/$08.00ϩ0 doi:10.1128/EC.5.1.148–154.2006 Copyright © 2006, American Society for Microbiology. All Rights Reserved. Pyruvate-Phosphate Dikinase of Oxymonads and Parabasalia and the Evolution of Pyrophosphate-Dependent Glycolysis in Anaerobic Eukaryotes† Claudio H. Slamovits and Patrick J. Keeling* Canadian Institute for Advanced Research, Botany Department, University of British Columbia, 3529-6270 University Boulevard, Vancouver, British Columbia V6T 1Z4, Canada Received 29 September 2005/Accepted 8 November 2005 In pyrophosphate-dependent glycolysis, the ATP/ADP-dependent enzymes phosphofructokinase (PFK) and pyruvate kinase are replaced by the pyrophosphate-dependent PFK and pyruvate phosphate dikinase (PPDK), respectively. This variant of glycolysis is widespread among bacteria, but it also occurs in a few parasitic anaerobic eukaryotes such as Giardia and Entamoeba spp. We sequenced two genes for PPDK from the amitochondriate oxymonad Streblomastix strix and found evidence for PPDK in Trichomonas vaginalis and other parabasalia, where this enzyme was thought to be absent. The Streblomastix and Giardia genes may be related to one another, but those of Entamoeba and perhaps Trichomonas are distinct and more closely related to bacterial homologues. These findings suggest that pyrophosphate-dependent glycolysis is more widespread in eukaryotes than previously thought, enzymes from the pathway coexists with ATP-dependent more often than previously thought and may be spread by lateral transfer of genes for pyrophosphate-dependent enzymes from bacteria. Adaptation to anaerobic metabolism is a complex process (PPDK), respectively (for a comparison of these reactions, see involving changes to many proteins and pathways of critical reference 21).