Fluid Resuscitation in Trauma: What Are the Best Strategies and Fluids? G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recognizing When a Child's Injury Or Illness Is Caused by Abuse
U.S. Department of Justice Office of Justice Programs Office of Juvenile Justice and Delinquency Prevention Recognizing When a Child’s Injury or Illness Is Caused by Abuse PORTABLE GUIDE TO INVESTIGATING CHILD ABUSE U.S. Department of Justice Office of Justice Programs 810 Seventh Street NW. Washington, DC 20531 Eric H. Holder, Jr. Attorney General Karol V. Mason Assistant Attorney General Robert L. Listenbee Administrator Office of Juvenile Justice and Delinquency Prevention Office of Justice Programs Innovation • Partnerships • Safer Neighborhoods www.ojp.usdoj.gov Office of Juvenile Justice and Delinquency Prevention www.ojjdp.gov The Office of Juvenile Justice and Delinquency Prevention is a component of the Office of Justice Programs, which also includes the Bureau of Justice Assistance; the Bureau of Justice Statistics; the National Institute of Justice; the Office for Victims of Crime; and the Office of Sex Offender Sentencing, Monitoring, Apprehending, Registering, and Tracking. Recognizing When a Child’s Injury or Illness Is Caused by Abuse PORTABLE GUIDE TO INVESTIGATING CHILD ABUSE NCJ 243908 JULY 2014 Contents Could This Be Child Abuse? ..............................................................................................1 Caretaker Assessment ......................................................................................................2 Injury Assessment ............................................................................................................4 Ruling Out a Natural Phenomenon or Medical Conditions -

A Rare Case of Penetrating Trauma of Frontal Sinus with Anterior Table Fracture Himanshu Raval1*, Mona Bhatt2 and Nihar Gaur3
ISSN: 2643-4474 Raval et al. Neurosurg Cases Rev 2020, 3:046 DOI: 10.23937/2643-4474/1710046 Volume 3 | Issue 2 Neurosurgery - Cases and Reviews Open Access CASE REPORT Case Report: A Rare Case of Penetrating Trauma of Frontal Sinus with Anterior Table Fracture Himanshu Raval1*, Mona Bhatt2 and Nihar Gaur3 1 Department of Neurosurgery, NHL Municipal Medical College, SVP Hospital Campus, Gujarat, India Check for updates 2Medical Officer, CHC Dolasa, Gujarat, India 3GAIMS-GK General Hospital, Gujarat, India *Corresponding author: Dr. Himanshu Raval, Resident, Department of Neurosurgery, NHL Municipal Medical College, SVP Hospital Campus, Elisbridge, Ahmedabad, Gujarat, 380006, India, Tel: 942-955-3329 Abstract Introduction Background: Head injury is common component of any Road traffic accident (RTA) is the most common road traffic accident injury. Injury involving only frontal sinus cause of cranio-facial injury and involvement of frontal is uncommon and unique as its management algorithm is bone fractures are rare and constitute 5-9% of only fa- changing over time with development of radiological modal- ities as well as endoscopic intervention. Frontal sinus inju- cial trauma. The degree of association has been report- ries may range from isolated anterior table fractures causing ed to be 95% with fractures of the anterior table or wall a simple aesthetic deformity to complex fractures involving of the frontal sinuses, 60% with the orbital rims, and the frontal recess, orbits, skull base, and intracranial con- 60% with complex injuries of the naso-orbital-ethmoid tents. Only anterior table injury of frontal sinus is rare in pen- region, 33% with other orbital wall fractures and 27% etrating head injury without underlying brain injury with his- tory of unconsciousness and questionable convulsion which with Le Fort level fractures. -
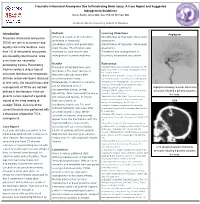
Traumatic Intracranial Aneurysms Due to Penetrating Brain Injury. a Case Report and Suggested Management Guidelines Breck Aaron Jones MD; Alex Patrick Michael MD
Traumatic Intracranial Aneurysms Due to Penetrating Brain Injury. A Case Report and Suggested Management Guidelines Breck Aaron Jones MD; Alex Patrick Michael MD Southern Illinois University School of Medicine Methods Learning Objectives Introduction Angiogram Traumatic intracranial aneurysms A Pubmed search of the literature Identification of traumatic intracranial pertaining to traumatic aneurysms. (TICA) are rare in occurrence and pseudoaneurysms and penetrating Classification of traumatic intracranial equally rare in the literature. Less brain trauma. The literature was aneurysms. than 1% of intracranial aneurysms reviewed for case reports and Treatment and management of are caused by blunt trauma, while management recommendations. traumatic intracranial aneurysms. even fewer are caused by penetrating trauma. Penetrating Results References Traumatic intracranial aneurysm 1.Aarabi B. Management of traumatic aneurysms caused by trauma creates a unique type of high-velocity missile head wounds. Neurosurg Clin N Am. formation is the most commonly aneurysm that does not incorporate Oct 1995;6(4):775-797. described vascular injury after 2.Rao GP, Rao NS, Reddy PK. Technique of removal of an all three vessel wall layers. Because penetrating brain injury. impacted sharp object in a penetrating head injury using the of their rarity, the natural history and Histologically, traumatic aneurysms lever principle. Br J Neurosurg. Dec 1998;12(6):569-571. 3.Vascular complications of penetrating brain injury. J can be described as true management of TICAs are not well Trauma. Aug 2001;51(2 Suppl):S26-28. Angiogram showing traumatic intracranial (incorporating intima, media, defined in the literature. Here we 4.Crompton MR. The pathogenesis of cerebral aneurysms. aneurysm following a gunshot wound to adventitia), false (incorporating one or Brain. -

Blunt and Blast Head Trauma: Different Entities
International Tinnitus Journal, Vol. 15, No. 2, 115–118 (2009) Blunt and Blast Head Trauma: Different Entities Michael E. Hoffer,1 Chadwick Donaldson,1 Kim R. Gottshall1, Carey Balaban,2 and Ben J. Balough1 1 Spatial Orientation Center, Department of Otolaryngology, Naval Medical Center San Diego, San Diego, California, and 2 Department of Otolaryngology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA Abstract: Mild traumatic brain injury (mTBI) caused by blast-related and blunt head trauma is frequently encountered in clinical practice. Understanding the nuances between these two distinct types of injury leads to a more focused approach by clinicians to develop better treat- ment strategies for patients. In this study, we evaluated two separate cohorts of mTBI patients to ascertain whether any difference exists in vestibular-ocular reflex (VOR) testing (n ϭ 55 en- rolled patients: 34 blunt, 21 blast) and vestibular-spinal reflex (VSR) testing (n ϭ 72 enrolled patients: 33 blunt, 39 blast). The VOR group displayed a preponderance of patients with blunt mTBI, demonstrating normal to high-frequency phase lag on rotational chair testing, whereas patients experiencing mTBI from blast-related causes revealed a trend toward low-frequency phase lag on evaluation. The VSR cohort showed that patients with posttraumatic migraine- associated dizziness tended to test higher on posturography. However, an indepth look at the total patient population in this second cohort reveals that a higher percentage of blast-exposed patients exhibited a significantly increased latency on motor control testing as compared to pa- tients with blunt head injury ( p Ͻ .02). These experiments identify a distinct difference be- tween blunt-injury and blast-injury mTBI patients and provide evidence that treatment strategies should be individualized on the basis of each mechanism of injury. -

Management of Acute Liver Failure In
Management of Acute Liver Failure in ICU Philip Berry MRCP, Clinical Research Fellow, Institute of Liver Studies, Kings College Hospital, London, UK Email: [email protected] Self assessment questions Scenario: A twenty-year-old female is brought into the Emergency Department having been found unconscious in her bedsit. There is no other recent history. She did not respond to a bolus of 50% dextrose in the ambulance, despite having an unrecordable blood glucose when tested by the paramedics. While she is being intubated on account of reduced level of consciousness, an arterial blood gas sample reveals profound lactic acidosis (pH 7.05, pCO2 2.5 kPa, base deficit – 10, lactate 13 mg/L). Blood pressure is 95/50 mmHg. 1. What are the possible explanations for her presentation? Laboratory tests demonstrate hepatocellular necrosis (AST 21,000 U/L) and coagulopathy (INR 9.1) with thrombocytopenia (platelet count 26 x 109/L). Acute liver failure appears the most likely diagnosis. 2. What are the most likely causes of acute liver failure (ALF) in this previously well patient? Her mean arterial blood pressure remains low (50mmHg) after 3 litres of colloid and crystalloid. The casualty nurse, who is doing half-hourly neurological observations, reports reduced pupillary response to light. 3. What severe complications of ALF may result in death within hours, and what are the immediate management priorities for this patient? Introduction Successful management of this rare but potentially devastating disorder relies on early recognition. The hallmark of acute liver failure (ALF) is encephalopathy (ranging from a subtle alterations in consciousness level to coma) in the context of an acute, severe liver injury. -

Delayed Traumatic Hemothorax in Older Adults
Open access Brief report Trauma Surg Acute Care Open: first published as 10.1136/tsaco-2020-000626 on 8 March 2021. Downloaded from Complication to consider: delayed traumatic hemothorax in older adults Jeff Choi ,1 Ananya Anand ,1 Katherine D Sborov,2 William Walton,3 Lawrence Chow,4 Oscar Guillamondegui,5 Bradley M Dennis,5 David Spain,1 Kristan Staudenmayer1 ► Additional material is ABSTRACT very small hemothoraces rarely require interven- published online only. To view, Background Emerging evidence suggests older adults tion whereas larger hemothoraces often undergo please visit the journal online immediate drainage. However, emerging evidence (http:// dx. doi. org/ 10. 1136/ may experience subtle hemothoraces that progress tsaco- 2020- 000626). over several days. Delayed progression and delayed suggests HTX in older adults with rib fractures may development of traumatic hemothorax (dHTX) have not experience subtle hemothoraces that progress in a 1Surgery, Stanford University, been well characterized. We hypothesized dHTX would delayed fashion over several days.1 2 If true, older Stanford, California, USA be infrequent but associated with factors that may aid adults may be at risk of developing empyema or 2Vanderbilt University School of Medicine, Nashville, Tennessee, prediction. other complications without close monitoring. USA Methods We retrospectively reviewed adults aged ≥50 Delayed progression and delayed development of 3Radiology, Vanderbilt University years diagnosed with dHTX after rib fractures at two traumatic hemothorax (dHTX) have not been well Medical Center, Nashville, level 1 trauma centers (March 2018 to September 2019). characterized in literature. The ageing US popula- Tennessee, USA tion and increasing incidence of rib fractures among 4Radiology, Stanford University, dHTX was defined as HTX discovered ≥48 hours after Stanford, California, USA admission chest CT showed either no or ’minimal/trace’ older adults underscore a pressing need for better 5Department of Surgery, HTX. -

Injury Surveillance Guidelines
WHO/NMH/VIP/01.02 DISTR.: GENERAL ORIGINAL: ENGLISH INJURY SURVEILLANCE GUIDELINES Edited by: Y Holder, M Peden, E Krug, J Lund, G Gururaj, O Kobusingye Designed by: Health & Development Networks http://www.hdnet.org Published in conjunction with the Centers for Disease Control and Prevention, Atlanta, USA, by the World Health Organization 2001 Copies of this document are available from: Injuries and Violence Prevention Department Non-communicable Diseases and Mental Health Cluster World Health Organization 20 Avenue Appia 1211 Geneva 27 Switzerland Fax: 0041 22 791 4332 Email: [email protected] The content of this document is available on the Internet at: http://www.who.int/violence_injury_prevention/index.html Suggested citation: Holder Y, Peden M, Krug E et al (Eds). Injury surveillance guidelines. Geneva, World Health Organization, 2001. WHO/NMH/VIP/01.02 © World Health Organization 2001 This document is not a formal publication of the World Health Organization (WHO). All rights are reserved by the Organization. The document may be freely reviewed, abstracted, reproduced or translated, in part or in whole, but may not be sold or used for commercial purposes. The views expressed in documents by named authors are the responsibility of those authors. ii Contents Acronyms .......................................................................................................................... vii Foreword .......................................................................................................................... viii Editorial -

Fluid Resuscitation in Trauma Patients: What Should We Know?
REVIEW CURRENT OPINION Fluid resuscitation in trauma patients: what should we know? Silvia Coppola, Sara Froio, and Davide Chiumello Purpose of review Fluid resuscitation in trauma patients could reduce organ failure, until blood components are available and hemorrhage is controlled. However, the ideal fluid resuscitation strategy in trauma patients remains a debated topic. Different types of trauma can require different types of fluids and different volume of infusion. Recent findings There are few randomized controlled trials investigating the efficacy of fluids in trauma patients. There is no evidence that any type of fluids can improve short-term and long-term outcome in these patients. The main clinical evidence emphasizes that a restrictive fluid resuscitation before surgery improves outcome in patients with penetrating trauma. Fluid management of blunt trauma patients, in particular with coexisting brain injury, remains unclear. Summary In order to focus on the state of the art about this topic, we review the current literature and guidelines. Recent studies have underlined that the correct fluid resuscitation strategy can depend on the type of trauma condition: penetrating, blunt, brain injury or a combination of them. Of course, further studies are needed to investigate the impact of a specific fluid strategy on different type and severity of trauma. Keywords blunt trauma, colloids, crystalloids, fluid resuscitation, penetrating trauma INTRODUCTION In the literature, there is little evidence that one type of fluid compared with another can improve Traumatic death is the main cause of life years lost & worldwide [1]. Hemorrhage is responsible for almost survival or can be more effective [4 ,6]. Few rando- 50% of deaths in the first 24 h after trauma [2,3]. -

Isolated Severe Blunt Traumatic Brain Injury: Effect of Obesity on Outcomes
CLINICAL ARTICLE J Neurosurg 134:1667–1674, 2021 Isolated severe blunt traumatic brain injury: effect of obesity on outcomes Jennifer T. Cone, MD, MHS,1 Elizabeth R. Benjamin, MD, PhD,2 Daniel B. Alfson, MD,2 and Demetrios Demetriades, MD, PhD2 1Department of Surgery, Section of Trauma and Acute Care Surgery, University of Chicago, Illinois; and 2Department of Surgery, Division of Trauma, Emergency Surgery, and Surgical Critical Care, LAC+USC Medical Center, University of Southern California, Los Angeles, California OBJECTIVE Obesity has been widely reported to confer significant morbidity and mortality in both medical and surgical patients. However, contemporary data indicate that obesity may confer protection after both critical illness and certain types of major surgery. The authors hypothesized that this “obesity paradox” may apply to patients with isolated severe blunt traumatic brain injuries (TBIs). METHODS The Trauma Quality Improvement Program (TQIP) database was queried for patients with isolated severe blunt TBI (head Abbreviated Injury Scale [AIS] score 3–5, all other body areas AIS < 3). Patient data were divided based on WHO classification levels for BMI: underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obesity class 1 (30.0–34.9 kg/m2), obesity class 2 (35.0–39.9 kg/m2), and obesity class 3 (≥ 40.0 kg/ m2). The role of BMI in patient outcomes was assessed using regression models. RESULTS In total, 103,280 patients were identified with isolated severe blunt TBI. Data were excluded for patients aged < 20 or > 89 years or with BMI < 10 or > 55 kg/m2 and for patients who were transferred from another treatment center or who showed no signs of life upon presentation, leaving data from 38,446 patients for analysis. -

Prehospital Spine Immobilization for Penetrating Trauma—Review and Recommendations from the Prehospital Trauma Life Support Executive Committee
REVIEW ARTICLE Prehospital Spine Immobilization for Penetrating Trauma—Review and Recommendations From the Prehospital Trauma Life Support Executive Committee Lance E. Stuke, MD, MPH, Peter T. Pons, MD, Jeffrey S. Guy, MD, MSc, MMHC, Will P. Chapleau, RN, EMT-P, Frank K. Butler, MD, Capt MC USN (Ret), and Norman E. McSwain, MD pine immobilization in trauma patients suspected of hav- In the case of penetrating injuries, delays in transport Sing a spinal injury has been a cornerstone of prehospital prolong the time before patients receive the prompt surgical treatment for decades. Current practices are based on the care needed to arrest hemorrhage. Even with experienced belief that a patient with an injured spinal column can prehospital providers, spine immobilization is time consum- deteriorate neurologically without immobilization. Most ing. The time required for experienced emergency medical treatment protocols do not differentiate between blunt and technicians to properly immobilize a cervical spine has been penetrating mechanisms of injury. Current Emergency Med- reported to be 5.64 minutes (Ϯ1.49 minutes).6 This scene ical Service (EMS) protocols for spinal immobilization of delay can be catastrophic for a patient with penetrating penetrating trauma are based on historic practices rather than trauma requiring urgent surgical intervention for airway com- scientific merits. Although blunt spinal column injuries will promise or hemorrhage. Studies have demonstrated that cervical collars increase occasionally produce unstable vertebral -

Validation of Lactate Clearance at 6 H for Mortality Prediction in Critically Ill Children
570 Research Article Validation of lactate clearance at 6 h for mortality prediction in critically ill children Rajeev Kumar, Nirmal Kumar Background and Aims: To validate the lactate clearance (LC) at 6 h for mortality Access this article online prediction in Pediatric Intensive Care Unit (PICU)-admitted patients and its comparison Website: www.ijccm.org with a pediatric index of mortality 2 (PIM 2) score. Design: A prospective, observational DOI: 10.4103/0972-5229.192040 study in a tertiary care center. Materials and Methods: Children <13 years of age, Quick Response Code: Abstract admitted to PICU were included in the study. Lactate levels were measured at 0 and 6 h of admission for clearance. LC and delayed or nonclearance group compared for in-hospital mortality and compared with PIM 2 score for mortality prediction. Results: Of the 140 children (mean age 33.42 months) who were admitted to PICU, 23 (16.42%) patients died. For LC cut-off (16.435%) at 6 h, 92 patients qualified for clearance and 48 for delayed or non-LC group. High mortality was observed (39.6%) in delayed or non-LC group as compared to clearance group (4.3%) (P = 0.000). LC cut-off of 16.435% at 6 h (sensitivity 82.6%, specificity 75.2%, positive predictive value 39.6%, and negative predictive value 95.7%) correlates with mortality. Area under receiver operating characteristic (ROC) for LC at 6 h for mortality prediction was 0.823 (P = 0.000). The area under ROC curve for expected mortality prediction by PIM 2 score at admission was 0.906 and at 12.3% cut-off of PIM 2 Score was related with mortality. -

Physical Injury, PTSD Symptoms, and Medication Use: Examination in Two Trauma Types
Journal of Traumatic Stress February 2014, 27, 74–81 Physical Injury, PTSD Symptoms, and Medication Use: Examination in Two Trauma Types Meghan W. Cody and J. Gayle Beck Department of Psychology, University of Memphis, Memphis, Tennessee, USA Physical injury is prevalent across many types of trauma experiences and can be associated with posttraumatic stress disorder (PTSD) symptoms and physical health effects, including increased medication use. Recent studies suggest that PTSD symptoms may mediate the effects of traumatic injury on health outcomes, but it is unknown whether this finding holds for survivors of different types of traumas. The current study examined cross-sectional relationships between injury, PTSD, and pain and psychiatric medication use in 2 trauma- exposed samples, female survivors of motor vehicle accidents (MVAs; n = 315) and intimate partner violence (IPV; n = 167). Data were obtained from participants at 2 trauma research clinics who underwent a comprehensive assessment of psychopathology following the stressor. Regression with bootstrapping suggested that PTSD symptoms mediate the relationship between injury severity and use of pain medications, R2 = .11, F(2, 452) = 28.37, p < .001, and psychiatric medications, R2 = .06, F(2, 452) = 13.18, p < .001, as hypothesized. Mediation, however, was not moderated by trauma type (ps > .05). Results confirm an association between posttraumatic psychopathology and medication usage and suggest that MVA and IPV survivors alike may benefit from assessment and treatment of emotional distress after physical injury. In a recent year, 45.4 million injury-related visits were re- ical health plays in recovery from injury (van der Kolk, Roth, ported at U.S.