Marine Science Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tribes and Empire on the Margins of Nineteenth-Century Iran
publications on the near east publications on the near east Poetry’s Voice, Society’s Song: Ottoman Lyric The Transformation of Islamic Art during Poetry by Walter G. Andrews the Sunni Revival by Yasser Tabbaa The Remaking of Istanbul: Portrait of an Shiraz in the Age of Hafez: The Glory of Ottoman City in the Nineteenth Century a Medieval Persian City by John Limbert by Zeynep Çelik The Martyrs of Karbala: Shi‘i Symbols The Tragedy of Sohráb and Rostám from and Rituals in Modern Iran the Persian National Epic, the Shahname by Kamran Scot Aghaie of Abol-Qasem Ferdowsi, translated by Ottoman Lyric Poetry: An Anthology, Jerome W. Clinton Expanded Edition, edited and translated The Jews in Modern Egypt, 1914–1952 by Walter G. Andrews, Najaat Black, and by Gudrun Krämer Mehmet Kalpaklı Izmir and the Levantine World, 1550–1650 Party Building in the Modern Middle East: by Daniel Goffman The Origins of Competitive and Coercive Rule by Michele Penner Angrist Medieval Agriculture and Islamic Science: The Almanac of a Yemeni Sultan Everyday Life and Consumer Culture by Daniel Martin Varisco in Eighteenth-Century Damascus by James Grehan Rethinking Modernity and National Identity in Turkey, edited by Sibel Bozdog˘an and The City’s Pleasures: Istanbul in the Eigh- Res¸at Kasaba teenth Century by Shirine Hamadeh Slavery and Abolition in the Ottoman Middle Reading Orientalism: Said and the Unsaid East by Ehud R. Toledano by Daniel Martin Varisco Britons in the Ottoman Empire, 1642–1660 The Merchant Houses of Mocha: Trade by Daniel Goffman and Architecture in an Indian Ocean Port by Nancy Um Popular Preaching and Religious Authority in the Medieval Islamic Near East Tribes and Empire on the Margins of Nine- by Jonathan P. -

Homemade Spices to Help You Save Time and Money
36 Homemade Spices to Help You Save Time and Money POULTRY SEASONING GREEK SEASONING 1 T rosemary; 1 T oregano; 1 T ground sage; 1 tsp. ¼ C dried oregano leaves; 2 TBS fennel seeds; 2 powdered ginger; 1 tsp. marjoram; 1 ½ tsp. thyme; 3 T TBS crushed dried lemon grass; ¾ tsp. black pepper packed brown sugar; 3 T dry minced parsley; 1 t. pepper; 1 T paprika; 2 T garlic salt; 2 T onion salt; 2 T chicken HERB MIX bouillon powder; 1 pkg. Lipton cup tomato soup mix Mix together in a mortar and pestle and then store 1 T onion powder; 1 T garlic powder; 1 T dried in an airtight container. When ready to use, mix 1 parsley flakes; 1 t. dried basil leaves; 1 t. dried tablespoon to 1 cup of flour for coating on any type thyme leaves; 1 t. dried marjoram leaves; 1 t. of pultry such as chicken, turkey, and cornish game pepper hens. HERBS DE PROVENCE CHILI POWDER 1 T dried basil leaves; 1 T dried marjoram leaves; 1 3 T paprika; 1 T ground cumin; 2 T oregano; 1 t. red T dried summer savory leaves; 1 T dried thyme or cayenne pepper; ½ tsp. garlic powder leaves; 2 t. orange zest; 1 powdered bay leaf; 1 t. fennel seeds CINNAMON SUGAR BREAKFAST SAUSAGE SEASONING 1 c granulated sugar; 2 T ground cinnamon 1 teaspoon Celtic sea salt; 1 teaspoon chili powder CREOLE SEASONING blend; ½ teaspoon dried thyme; ½ teaspoon fennel seed; ½ teaspoon ground ginger; ¼ teaspoon onion 4 t. salt; 4 t. paprika; 1 T garlic powder; 1 T black powder; ¼ teaspoon dried oregano; ¼ teaspoon pepper; 2 ½ t. -

Copyright by Mohammad Raisur Rahman 2008
Copyright by Mohammad Raisur Rahman 2008 The Dissertation Committee for Mohammad Raisur Rahman certifies that this is the approved version of the following dissertation: Islam, Modernity, and Educated Muslims: A History of Qasbahs in Colonial India Committee: _____________________________________ Gail Minault, Supervisor _____________________________________ Cynthia M. Talbot _____________________________________ Denise A. Spellberg _____________________________________ Michael H. Fisher _____________________________________ Syed Akbar Hyder Islam, Modernity, and Educated Muslims: A History of Qasbahs in Colonial India by Mohammad Raisur Rahman, B.A. Honors; M.A.; M.Phil. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin August 2008 Dedication This dissertation is dedicated to the fond memories of my parents, Najma Bano and Azizur Rahman, and to Kulsum Acknowledgements Many people have assisted me in the completion of this project. This work could not have taken its current shape in the absence of their contributions. I thank them all. First and foremost, I owe my greatest debt of gratitude to my advisor Gail Minault for her guidance and assistance. I am grateful for her useful comments, sharp criticisms, and invaluable suggestions on the earlier drafts, and for her constant encouragement, support, and generous time throughout my doctoral work. I must add that it was her path breaking scholarship in South Asian Islam that inspired me to come to Austin, Texas all the way from New Delhi, India. While it brought me an opportunity to work under her supervision, I benefited myself further at the prospect of working with some of the finest scholars and excellent human beings I have ever known. -

HCG FUSION RECIPES Stevia and Truvia- Natural Sweeteners Stevia Is an Herb That Has Been Used As a Sweetener for Hundreds of Years
HCG FUSION RECIPES Stevia and Truvia- Natural Sweeteners Stevia is an herb that has been used as a sweetener for hundreds of years. The leaves of this small plant have a delicious and refreshing taste. Stevia will not raise your blood pressure, it may actually lower blood sugar levels. Truvia a natural sweetener is made from rebiana, the best tasting part of the stevia leaf. Truvia is a zero calorie sweetner. DRESSINGS Viniaigrette ¼ cup apple cider vinegar 1 dash of onion powder 2 packets of Stevia ½ cup water ¼ t. sea salt 2 dashes of celery salt Pepper to taste 1 T. lemon juice Chipolte Vinaigrette Same as above just add- 1/8 t. paprika, 1/8 t. nutmeg, 1/8 t. cinnamon, ¼ t. chipotle seasoning, dash of garlic and Onion powder. Italian Dressing 1 cup of water 1/2 cup apple cider vinegar ½ t. sea salt ½ t. Italian seasoning 1 t. onion powder 2 packets Stevia SALADS Cucumber Salad 2 t. fresh basil, chopped 2 cucumbers, peeled 2 t. apple cider vinegar ½ t. lemon juice Sea Salt to taste Pepper to taste Combine ingredients together and chill for 2 hours. Curry Chicken Salad Chicken breast(chopped) Celery 1 apple(diced) 2 t. lemon juice 1 minced garlic clove Dash of cinnamon, turmeric, onion powder, garlic powder, Cayenne pepper Use 100 grams chicken breast. Sautee chicken, water, and lemon juice, add spices and stir well. Simmer over low heat. Chicken Salad Chicken Breast (chopped) 2 Melba toast Viniagrette dressing ¼ cup chicken broth Lettuce Weigh chicken breast to 100 grams, add salt and pepper. -

Norsk Varemerketidende Nr 47/15
. nr 47/15 - 2015.11.16 NO årgang 105 ISSN 1503-4925 Norsk varemerketidende er en publikasjon som inneholder kunngjøringer innenfor varemerkeområdet BESØKSADRESSE f Sandakerveien 64 POSTADRESSE f Postboks 8160 Dep. 0033 Oslo E-POST f [email protected] TELEFON f 22 38 73 00 TELEFAKS f 22 38 73 01 INFOSENTERETS TELEFONTID f kl. 09.00 - 15.00 Telefon (+47) 22 38 73 33 Telefaks (+47) 22 38 73 31 innholdsfortegnelse og inid-koder 2015.11.16 - 47/15 Innholdsfortegnelse: Registrerte varemerker ......................................................................................................................................... 3 Internasjonale varemerkeregistreringer ............................................................................................................ 46 Ansvarsmerker .................................................................................................................................................. 136 Innsigelser .......................................................................................................................................................... 137 Avgjørelser etter innsigelser ............................................................................................................................ 138 Avgjørelse etter krav om administrativ overprøving av varemerkeregistrering ......................................... 139 Avgjørelser fra Klagenemnda........................................................................................................................... 140 Begrensing -

Caste, Kinship and Sex Ratios in India
NBER WORKING PAPER SERIES CASTE, KINSHIP AND SEX RATIOS IN INDIA Tanika Chakraborty Sukkoo Kim Working Paper 13828 http://www.nber.org/papers/w13828 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 March 2008 We thank Bob Pollak, Karen Norberg, David Rudner and seminar participants at the Work, Family and Public Policy workshop at Washington University for helpful comments and discussions. We also thank Lauren Matsunaga and Michael Scarpati for research assistance and Cassie Adcock and the staff of the South Asia Library at the University of Chicago for their generous assistance in data collection. We are also grateful to the Weidenbaum Center and Washington University (Faculty Research Grant) for research support. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- reviewed or been subject to the review by the NBER Board of Directors that accompanies official NBER publications. © 2008 by Tanika Chakraborty and Sukkoo Kim. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Caste, Kinship and Sex Ratios in India Tanika Chakraborty and Sukkoo Kim NBER Working Paper No. 13828 March 2008 JEL No. J12,N35,O17 ABSTRACT This paper explores the relationship between kinship institutions and sex ratios in India at the turn of the twentieth century. Since kinship rules varied by caste, language, religion and region, we construct sex-ratios by these categories at the district-level using data from the 1901 Census of India for Punjab (North), Bengal (East) and Madras (South). -

Maasir-I-'Alamgiri
-BIBLIOTHECA INDICA MAASIR-I-'ALAMGIRI A History of the EmperorL.turangzib-' Xlamgir (reign 1658-1707 A.D.) OF ,?AQI MUST'AD KHAN Translated into English and annotated by SIR JADU-NATH SARKAR, Kt., C.I.E., Hony. D.Utt., M.A., I.E.s. (ret.), Honorary Member, Royal Asiatic Society 0/ Great Britain and Ireland; Corresponding Member, Royal Historical Society, Honorary Fe!!ow, Royal Asiatic SOCiety of Bengal, and of Bombay Branen, Royal Asiatic Society of G. B. CAI.cu't'tA : ROYAL ASIArIC SOCIaT\" OF BENGAL. I PARK STREET. 1947 Work No. 269. Issue No. 1556. P,ies Rs. 10/. \12,(;.1.-0 7 l-{ 7 i \O~53y, Printed by P. C. Ray, at Sri Gouranga PresS, Ii <;;h~laJ.llan.i 1}as tan~. Calcutta. TRANSLATOR'S PREFACE l THJt Emperor Akbar (reign 1556-1605 A.D.) set the example of having a detailed history of his reign written by official command. The result was the A~~1!'!g;h or 'Book of Akbar' of. ~uly-)y:l (completed by other hands after that author's death). Then came the Emperor Jahangir" who dictated his own memoirs, known as the Tuzuk-i-Jahangiri, and therefore no official Jahiingir-niimah had to be written about him. This book, however, combines the literary characteristics of an autobiography with those of an official history, or in other words, it gives the Emperor's own reflections and feelings as well as an objective record of the events of his reign. Indeed, in this reign, the literary type of the Mughal official histories was determined for the future, as was exemplified by the Piidishiihniimah (Shah Jahan), the (Alamgir-1Ui.mah (of Aurangzib, completed by the Maasir-i-Alamgiri), the Bahadur Shah-niimah (Shah Alam I) and later attempts like the Tiirikh-i-Ahmad Shahi and the Tiirikh-i-(Alamgir $iini. -

Parke Happenings
April 2017 It’s More Than a Community. It’s a Lifestyle. The Board of Directors Corner Annual Meeting and Board From Director Jim Angstadt Election coming May 4 The Parke at Ocean Pines Annual Meeting and Board On May 4, I will complete my sixth year of service on the Election will be held on Thursday, May 4, at 2:30pm in the Board of Directors of this great community. Since 2006, when Clubhouse Grand Hall. The Nominating/Election Committee I first became involved with the workings of our HOA, I have has nominated three candidates to fill the seats of two direc- participated with other homeowners as a member of several tors whose terms expire on May 5. The Annual Meeting pack- advisory committees as well as on the Board. It has been edu- et will be mailed to each lot owner’s address on file Friday, cational, fun, and indeed a privilege to have served with many March 31. The packet will contain the voting instructions and each of the three candidate’s nomination submission. Please volunteers whose common goal is to work for the betterment be sure to set aside time to vote as the voting will begin on of our community. Monday, April 3, and end Monday, May 1, at 5:00pm. The My hope is for folks to continue to serve on committees Homeowners Association will use the same electronic voting and on the Board of Directors so that our residents are driv- website as the previous three years as well as a paper ballot ing the decisions made about budgets, facilities, rules, and the for those who prefer to vote manually. -

Guide #2 - Infused Salts
Guide #2 - Infused Salts Infused Salts are created by using only the finest sea this salt has a spicy hot flavor that is without compare. Named for one of salts, and then mixing them with raw, all natural in- the most venomous snakes in India, this pepper, also known as the King gredients. The mixtures are then allowed to evaporate Cobra Chili, has a blazing heat that is out of this world. Uses: For some flaming flavor, sprinkle over chicken wings, chili, or your fajitas. or are heated until the crystals reform. This creates a uniform flavor throughout the salt grains. This is also Habanero Heat Sea Salt why some salts are sticky and clumpy. This is a good Ground down, high-quality, high-heat habanero peppers create an thing! Theses salts are not intended to be shaken out of intense sea salt with a kick. This is the perfect salt for anyone who likes to a bottle. Instead they are meant to enjoyedthe old fash- add a little extra punch to anything on the grill. Uses: A great match with ioned way. Pinched these salts onto the food, or use a recipes that call for lime and butter. Use sparingly! small salt spoon. Break them apart with a fork if neces- Jalapeno Sea Salt sary. Infused salts are 100% natural and gluten free. A blend of all natural sea salt and authentic jalapeno pepper. This salt will give a bite of spice to your favorite recipes on the grill or in the kitchen. Uses: Heat up guacamole, flank steak, and chicken and rice for a kick. -
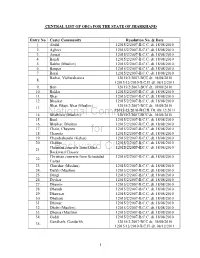
1 CENTRAL LIST of Obcs for the STATE of JHARKHAND Entry No
CENTRAL LIST OF OBCs FOR THE STATE OF JHARKHAND Entry No Caste/ Community Resolution No. & Date 1. Abdal 12015/2/2007-B.C.C. dt. 18/08/2010 2. Aghori 12015/2/2007-B.C.C. dt. 18/08/2010 3. Amaat 12015/2/2007-B.C.C. dt. 18/08/2010 4. Bagdi 12015/2/2007-B.C.C. dt. 18/08/2010 5. Bakho (Muslim) 12015/2/2007-B.C.C. dt. 18/08/2010 6. Banpar 12015/2/2007-B.C.C. dt. 18/08/2010 7. Barai 12015/2/2007-B.C.C. dt. 18/08/2010 Barhai, Vishwakarma 12015/2/2007-BCC dt. 18/08/2010 8. 12015/13/2010-B.C.II. dt. 08/12/2011 9. Bari 12015/2/2007-BCC dt. 18/08/2010 10. Beldar 12015/2/2007-B.C.C. dt. 18/08/2010 11. Bhar 12015/2/2007-B.C.C. dt. 18/08/2010 12. Bhaskar 12015/2/2007-B.C.C. dt. 18/08/2010 Bhat, Bhatt, Bhat (Muslim) 12015/2/2007-BCC dt. 18/08/2010 13. 12015/13/2010-B.C.II. Dt. 08/12/2011 14. Bhathiara (Muslim) 12015/2/2007-BCC dt. 18/08/2010 15. Bind 12015/2/2007-B.C.C. dt. 18/08/2010 16. Bhuihar, Bhuiyar 12015/2/2007-B.C.C. dt. 18/08/2010 17. Chain, Chayeen 12015/2/2007-B.C.C. dt. 18/08/2010 18. Chapota 12015/2/2007-B.C.C. dt. 18/08/2010 19. Chandrabanshi (Kahar) 12015/2/2007-B.C.C. -

Multi-Purpose Blends Description Seasoned Salt
The Valdez Corporation - Product List Multi-Purpose Blends Description Seasoned Salt -L #805 Lawry's type, heavy salt Seasoned Salt #368 typical all-purpose with a celery note Seasoned Salt #3840 all-purpose with a chili pepper note Lemon Pepper #678 strong lemon, bright yellow color Seasoned Salt - No MSG #4923 typical all purpose with no MSG Lemon Pepper #2056 heavy salt, strong acidic note Lemon Pepper #353 strong garlic w/ large pepper Lemon Pepper #314 very tart, heavy pepper Garlic Pepper #348 traditional garlic pepper, all-purpose Garlic Pepper #1113 lower salt version of #348 Roasted Garlic Pepper #1215 all-purpose with a roasted note Seasoned Pepper #4931 strong peppery notes Garlic Salt #975 with granulated garlic Onion Salt #1073 with granulated onion Celery Salt #246 traditional blend of salt & celery Bay Seasoning #264 all-purpose blend with celery flavor New Bay Seasoning #3191 paprika based with some heat Bay Seasoning #4924 earthy type flavor - great for seafood Original Chicken Seasoning RD 6360-A great all purpose seasoning Poultry Seasoning #363 traditional with strong sage Spike Type Seasoning #734 unique all-purpose blend with savory notes Salt-Free Dash Seasoning #142 all-purpose with heavy herbs & spices Peppercorn Medley #313 whole pink, green, and black peppercorns Herbs of Provence #586 dry herb blend with lavender Sticky Corn Seasoning #4496 sweet and salty with low heat Tequila Lime Seasoning #4434 tangy blend with a unique taste Tequila Lime w/Jalapeno Seasoning #4960 tangy blend with a unique kick Cilantro -

Ethnohistory of the Qizilbash in Kabul: Migration, State, and a Shi'a Minority
ETHNOHISTORY OF THE QIZILBASH IN KABUL: MIGRATION, STATE, AND A SHI’A MINORITY Solaiman M. Fazel Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements for the degree Doctor of Philosophy in the Department of Anthropology Indiana University May 2017 i Accepted by the Graduate Faculty, Indiana University, in partial fulfillment of the requirement for the degree of Doctor of Philosophy. Doctoral Committee __________________________________________ Raymond J. DeMallie, PhD __________________________________________ Anya Peterson Royce, PhD __________________________________________ Daniel Suslak, PhD __________________________________________ Devin DeWeese, PhD __________________________________________ Ron Sela, PhD Date of Defense ii For my love Megan for the light of my eyes Tamanah and Sohrab and for my esteemed professors who inspired me iii ACKNOWLEDGEMENT This historical ethnography of Qizilbash communities in Kabul is the result of a painstaking process of multi-sited archival research, in-person interviews, and collection of empirical data from archival sources, memoirs, and memories of the people who once live/lived and experienced the affects of state-formation in Afghanistan. The origin of my study extends beyond the moment I had to pick a research topic for completion of my doctoral dissertation in the Department of Anthropology, Indiana University. This study grapples with some questions that have occupied my mind since a young age when my parents decided to migrate from Kabul to Los Angeles because of the Soviet-Afghan War of 1980s. I undertook sections of this topic while finishing my Senior Project at UC Santa Barbara and my Master’s thesis at California State University, Fullerton. I can only hope that the questions and analysis offered here reflects my intellectual progress.