Acoustic Trapping of Sub-Micrometre Particles Within Microfluidics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNIT 1 ELECTROMAGNETIC RADIATION Radiation
Electromagnetic UNIT 1 ELECTROMAGNETIC RADIATION Radiation Structure 1.1 Introduction Objectives 1.2 What is Electromagnetic Radiation? Wave Mechanical Model of Electromagnetic Radiation Quantum Model of Electromagnetic Radiation 1.3 Consequences of Wave Nature of Electromagnetic Radiation Interference Diffraction Transmission Refraction Reflection Scattering Polarisation 1.4 Interaction of EM Radiation with Matter Absorption Emission Raman Scattering 1.5 Summary 1.6 Terminal Questions 1.7 Answers 1.1 INTRODUCTION You would surely have seen a beautiful rainbow showing seven different colours during the rainy season. You know that this colourful spectrum is due to the separation or dispersion of the white light into its constituent parts by the rain drops. The rainbow spectrum is just a minute part of a much larger continuum of the radiations that come from the sun. These are called electromagnetic radiations and the continuum of the electromagnetic radiations is called the electromagnetic spectrum. In the first unit of this course you would learn about the electromagnetic radiation in terms of its nature, characteristics and properties. Spectroscopy is the study of interaction of electromagnetic radiation with matter. We would discuss the ways in which different types of electromagnetic radiation interact with matter and also the types of spectra that result as a consequence of the interaction. In the next unit you would learn about ultraviolet-visible spectroscopy ‒ a consequence of interaction of electromagnetic radiation in the ultraviolet-visible -

Realization of a Micrometre-Sized Stochastic Heat Engine Valentin Blickle1,2* and Clemens Bechinger1,2
Erschienen in: Nature Physics ; 8 (2012), 2. - S. 143-146 https://dx.doi.org/10.1038/nphys2163 Realization of a micrometre-sized stochastic heat engine Valentin Blickle1,2* and Clemens Bechinger1,2 The conversion of energy into mechanical work is essential trapping centre and k(t), the time-dependent trap stiffness. The for almost any industrial process. The original description value of k is determined by the laser intensity, which is controlled of classical heat engines by Sadi Carnot in 1824 has largely by an acousto-optic modulator. By means of video microscopy, the shaped our understanding of work and heat exchange during two-dimensional trajectory was sampled with spatial and temporal macroscopic thermodynamic processes1. Equipped with our resolutions of 10 nm and 33 ms, respectively. Keeping hot and cold present-day ability to design and control mechanical devices at reservoirs thermally isolated at small length scales is experimentally micro- and nanometre length scales, we are now in a position very difficult to achieve, so rather than coupling our colloidal to explore the limitations of classical thermodynamics, arising particle periodically to different heat baths, here we suddenly on scales for which thermal fluctuations are important2–5. Here changed the temperature of the surrounding liquid. we demonstrate the experimental realization of a microscopic This variation of the bath temperature was achieved by a second heat engine, comprising a single colloidal particle subject to a coaxially aligned laser beam whose wavelength was matched to an time-dependent optical laser trap. The work associated with absorption peak of water. This allowed us to heat the suspension the system is a fluctuating quantity, and depends strongly from room temperature to 90 ◦C in less than 10 ms (ref. -

Hybrid Electrochemical Capacitors
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 1-11-2018 Hybrid Electrochemical Capacitors: Materials, Optimization, and Miniaturization Richa Agrawal Department of Mechanical and Materials Engineering, Florida International University, [email protected] DOI: 10.25148/etd.FIDC006581 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Materials Chemistry Commons, Materials Science and Engineering Commons, Nanoscience and Nanotechnology Commons, and the Physical Chemistry Commons Recommended Citation Agrawal, Richa, "Hybrid Electrochemical Capacitors: Materials, Optimization, and Miniaturization" (2018). FIU Electronic Theses and Dissertations. 3680. https://digitalcommons.fiu.edu/etd/3680 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida HYBRID ELECTROCHEMICAL CAPACITORS: MATERIALS, OPTIMIZATION, AND MINIATURIZATION A dissertation submitted in partial fulfillment of the requirement for the degree of DOCTOR OF PHILOSOPHY in MATERIALS SCIENCE AND ENGINEERING by Richa Agrawal 2018 To: Dean John L. Volakis College of Engineering and Computing This dissertation entitled Hybrid Electrochemical Capacitors: Materials, Optimization, and Miniaturization, written -

A Comparative Study of Jadeite, Omphacite and Kosmochlor Jades from Myanmar, and Suggestions for a Practical Nomenclature
Feature Article A Comparative Study of Jadeite, Omphacite and Kosmochlor Jades from Myanmar, and Suggestions for a Practical Nomenclature Leander Franz, Tay Thye Sun, Henry A. Hänni, Christian de Capitani, Theerapongs Thanasuthipitak and Wilawan Atichat Jadeitite boulders from north-central Myanmar show a wide variability in texture and mineral content. This study gives an overview of the petrography of these rocks, and classiies them into ive different types: (1) jadeitites with kosmochlor and clinoamphibole, (2) jadeitites with clinoamphibole, (3) albite-bearing jadeitites, (4) almost pure jadeitites and (5) omphacitites. Their textures indicate that some of the assemblages formed syn-tectonically while those samples with decussate textures show no indication of a tectonic overprint. Backscattered electron images and electron microprobe analyses highlight the variable mineral chemistry of the samples. Their extensive chemical and textural inhomogeneity renders a classiication by common gemmological methods rather dificult. Although a deinitive classiication of such rocks is only possible using thin-section analysis, we demonstrate that a fast and non-destructive identiication as jadeite jade, kosmochlor jade or omphacite jade is possible using Raman and infrared spectroscopy, which gave results that were in accord with the microprobe analyses. Furthermore, current classiication schemes for jadeitites are reviewed. The Journal of Gemmology, 34(3), 2014, pp. 210–229, http://dx.doi.org/10.15506/JoG.2014.34.3.210 © 2014 The Gemmological Association of Great Britain Introduction simple. Jadeite jade is usually a green massive The word jade is derived from the Spanish phrase rock consisting of jadeite (NaAlSi2O6; see Ou Yang, for piedra de ijada (Foshag, 1957) or ‘loin stone’ 1999; Ou Yang and Li, 1999; Ou Yang and Qi, from its reputed use in curing ailments of the loins 2001). -

Orders of Magnitude (Length) - Wikipedia
03/08/2018 Orders of magnitude (length) - Wikipedia Orders of magnitude (length) The following are examples of orders of magnitude for different lengths. Contents Overview Detailed list Subatomic Atomic to cellular Cellular to human scale Human to astronomical scale Astronomical less than 10 yoctometres 10 yoctometres 100 yoctometres 1 zeptometre 10 zeptometres 100 zeptometres 1 attometre 10 attometres 100 attometres 1 femtometre 10 femtometres 100 femtometres 1 picometre 10 picometres 100 picometres 1 nanometre 10 nanometres 100 nanometres 1 micrometre 10 micrometres 100 micrometres 1 millimetre 1 centimetre 1 decimetre Conversions Wavelengths Human-defined scales and structures Nature Astronomical 1 metre Conversions https://en.wikipedia.org/wiki/Orders_of_magnitude_(length) 1/44 03/08/2018 Orders of magnitude (length) - Wikipedia Human-defined scales and structures Sports Nature Astronomical 1 decametre Conversions Human-defined scales and structures Sports Nature Astronomical 1 hectometre Conversions Human-defined scales and structures Sports Nature Astronomical 1 kilometre Conversions Human-defined scales and structures Geographical Astronomical 10 kilometres Conversions Sports Human-defined scales and structures Geographical Astronomical 100 kilometres Conversions Human-defined scales and structures Geographical Astronomical 1 megametre Conversions Human-defined scales and structures Sports Geographical Astronomical 10 megametres Conversions Human-defined scales and structures Geographical Astronomical 100 megametres 1 gigametre -

1.0 GENERAL 1.1 Abbreviation/Symbol Meaning Μm Micrometre Or Micron Mm Millimetre M Metre Mm Or Mm2 Square Millimetre M Or M2 S
Section 01275 Alberta Transportation Measurement Rules Tender No. [ ] Page 1 1.0 GENERAL 1.1 MEASUREMENT SYSTEM .1 This section specifies the measurement rules that will generally be used for payment purposes unless otherwise specified in the Contract Documents. In case of conflict between the method of measurement specified in this section and the requirements specified in Section 01280 – Measurement Schedule, the latter will govern. .2 Work will be measured in the International System of Units (SI) in accordance with CAN/CSA–Z234.1–89 Canadian Metric Practice Guide. .3 When used in the Contract, the following abbreviations and symbols have the meaning assigned to them. Abbreviation/Symbol Meaning µm micrometre or micron mm millimetre m metre mm2 or mm2 square millimetre m2 or m2 square metre ha hectare kPa kilopascal MPa megapascal m3 or m3 cubic metre l (or where clarity is needed L) litre L.S. lump sum g gram kg kilogram N newton kN kilonewton t tonne no. number (quantity) min minute (time) h hour d day wk week % percent > greater than greater than or equal to < less than less than or equal to $ Canadian dollars ° degree (angle) °C degree Celsius Section 01275 Alberta Transportation Measurement Rules Tender No. [ ] Page 2 1.2 METHOD OF MEASUREMENT .1 Unless otherwise indicated in the Contract Documents: .1 earthwork materials will be measured net in place after compaction, with no allowance for bulking, shrinkage, compression, foundation settlement, or waste; .2 products will be measured net, with no allowance for waste; .3 dimensions used in calculating quantities will be rounded to the nearest unit of dimension as follows: Quantity Dimension [Volume of earth centimetre Volume of concrete millimetre Length of pipe centimetre Area of land decimetre [ ] [ ]] .4 the survey [station line] [station grid] system adopted will be at [10] [15] [20] [30] [100] linear metres spacing for measuring [ ], respectively; .5 contours may be based on aerial photograph interpretation and are approximate only. -

GEA Breconcherry Cleaning Technology Business Line Cleaning Technology Catalog 2014
GEA Breconcherry Cleaning Technology Business Line Cleaning Technology Catalog 2014 engineering for a better world GEA Mechanical Equipment Publication date: 01 / 01 / 2014 The article numbers and technical specifications are up to date. The publication of specifications, technical data and information in written or electronic form does not release the user from the responsibility of checking for themselves all products delivered by us for suitability for the application(s) intended. These may be subject to change without prior notification. Mistakes and printing errors excepted – we assume no liability for the correctness of specifications given. The general terms and conditions of delivery apply. All rights reserved - copyright on all contents: GEA Breconcherry GEA Mechanical Equipment UK Ltd Unit 4 Porthouse Business Centre, Tenbury Road, Bromyard, Herefordshire, HR7 4FL Registered office: Milton Keynes, Place of Registration: London, Reg. No. 611624 Management Board: Chairman Markus Hüllmann Managing Director: Barry Dumble VAT ID number: GB 792406809 GEA Breconcherry Cleaning components Table of contents for cleaning components and accessories Pages Introduction 5 - 9 Product selection tables 10 - 13 Resistance of sealing materials 14 Orbital cleaner - Twister 15 Orbital cleaner - Typhoon 16 Orbital cleaner - Tempest 17 Orbital cleaner - Tornado 18 Orbital cleaner - Tornado 4 19 Orbital cleaner - TMC 45/85 20 - 21 Rotating cleaner - Torrent 22 Rotating cleaner - Rotating Jet Cleaners 2E 23 - 27 Rotating cleaner - Turbo SSB 40, 75, -

Intensity Wave Length
Module 9 Radiative Transfer in the Atmosphere 9.1 Introduction It has been emphasized in earlier lectures that energy from Sun drives the circulation of the atmosphere and ocean. Earth receives energy from Sun as ultraviolet, visible and near-infrared radiation. This radiation band is called the short wave radiation or radiation with wavelengths λ < 4 µm . An equal amount of energy is re-emitted by earth to maintain an overall energy balance. Earth emits energy in the form of infrared thermal radiation or longwave radiation with wavelengths λ > 4 µm . These two bands shown in Fig. 9.1 pertain to the wavelength intervals given as, Band 1: Solar spectrum 0.1− 4 µm (1 µm = 10−6 m) Band 2: Infrared region 4 −100 µm Sun Earth Fig. 9.1 Blackbody emission at the temperatures of Sun and Earth. 6000 K 288 K Intensity Overlap region Overlap 0.1 0.5 1 5 10 50 100 Wave length ( µ m ) Microwave radiation is not important for energy balance of the earth, but it finds wide use in remote sensing of the earth system because it is capable of penetrating through clouds. The study of radiative transfer in atmosphere and ocean is important because it leads to (i) a better understanding of energy transfer in these two components of the climate system; and (ii) in interpreting remote sensing measurements from satellites. Radiation is described by flux, intensity and radiance; and there are certain terms also that are frequently used while discussing the mathematics of the radiative transfer. First of all, it is required to calculate a differential amount of radiant energy dEλ ( J ) in a wavelength ( µm ) interval λ and λ + dλ that crosses an area ( m2 ) element dA in time (seconds) interval dt in a direction confined by an arc dω of the solid angle (steradian abbreviated as sr ). -

Fundamental Good Practice in Dimensional Metrology
Good Practice Guide No. 80 Fundamental Good Practice in Dimensional Metrology David Flack and John Hannaford Measurement Good Practice Guide No. 80 Fundamental Good Practice in Dimensional Metrology David Flack Engineering Measurement Services Team Engineering Measurement Division John Hannaford ABSTRACT This good practice guide is written for those who need to make dimensional measurements but are not necessarily trained metrologists. On reading this guide you should have gained a basic knowledge of fundamental good practice when making dimensional measurements. An introduction to length units and key issues such as traceability and uncertainty is followed by some examples of typical sources of error in length measurement. Checking to specification, accreditation and measurement techniques are also covered along with an introduction to optical measurement techniques. © Queen's Printer and Controller of HMSO First printed July 2005 Reprinted with minor corrections/amendments October 2012 ISSN 1368-6550 National Physical Laboratory Hampton Road, Teddington, Middlesex, TW11 0LW Acknowledgements This document has been produced for the Department for Business, Innovation and Skills; National Measurement System under contract number GBBK/C/08/17. Thanks also to Hexagon Metrology, Romer, Renishaw and Faro UK for providing some of the images and to Dr Richard Leach (NPL), Simon Oldfield (NPL), Dr Anthony Gee (University College London) and Prof Derek Chetwynd (University of Warwick) for suggesting improvements to this guide. i Contents Introduction -
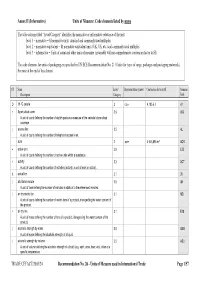
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Engilab Units 2018 V2.2
EngiLab Units 2021 v3.1 (v3.1.7864) User Manual www.engilab.com This page intentionally left blank. EngiLab Units 2021 v3.1 User Manual (c) 2021 EngiLab PC All rights reserved. No parts of this work may be reproduced in any form or by any means - graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems - without the written permission of the publisher. Products that are referred to in this document may be either trademarks and/or registered trademarks of the respective owners. The publisher and the author make no claim to these trademarks. While every precaution has been taken in the preparation of this document, the publisher and the author assume no responsibility for errors or omissions, or for damages resulting from the use of information contained in this document or from the use of programs and source code that may accompany it. In no event shall the publisher and the author be liable for any loss of profit or any other commercial damage caused or alleged to have been caused directly or indirectly by this document. "There are two possible outcomes: if the result confirms the Publisher hypothesis, then you've made a measurement. If the result is EngiLab PC contrary to the hypothesis, then you've made a discovery." Document type Enrico Fermi User Manual Program name EngiLab Units Program version v3.1.7864 Document version v1.0 Document release date July 13, 2021 This page intentionally left blank. Table of Contents V Table of Contents Chapter 1 Introduction to EngiLab Units 1 1 Overview .................................................................................................................................. -

Table of Contents
Table of Contents Teaching and Learning The Metric System Unit 1 1 - Suggested Teaching Sequence 1 - Objectives 1 - Rules of Notation 1 - Metric Units, Symbols, and Referents 2 - Metric Prefixes 2 - Linear Measurement Activities 3 - Area Measurement Activities 5 - Volume Measurement Activities 7 - Mass (Weight) Measurement Activities 9 - Temperature Measurement Activities 11 Unit 2 12 - Objectives 12 - Suggested Teaching Sequence 12 - Metrics in this Occupation 12 - Metric Units For Architectural, Civil and Mechanical Drafting - Trying Out Metric Units 13 - Building With Metrics 14 - Drafting With Metrics 15 - Drawing With Metrics 16 17 Unit 3 18 - Objective 18 - Suggested Teaching Sequence 18 - Metric-Metric Equivalents 18 - Changing Units at Work 20 Unit 4 21 - Objective 21 - Suggested Teaching Sequence 21 - Selecting and Using Metric Instruments, Tools and Devices 21 - Which Tools for the Job? 22 - Measuring Up in Architectural Drafting 22 - Which Tools for the Job? 23 - Measuring Up in Civil Drafting 23 - Which Tools for the Job? 24 - Measuring Up in Mechanical Drafting 24 Unit 5 25 - Objective 25 - Suggested Teaching Sequence 25 - Metric-Customary Equivalents 25 - Conversion Tables 26 - Any Way You Want It 27 Testing Metric Abilities 28 Answers to Exercises and Test 30 Tools and Devices List References TEACHING AND LEARNING THE METRIC SYSTEM This metric instructional package was designed to meet job-related Unit 2 provides the metric terms which are used in this occupation metric measurement needs of students. To use this package students and gives experience with occupational measurement tasks. should already know the occupational terminology, measurement terms, and tools currently in use. These materials were prepared with Unit 3 focuses on job-related metric equivalents and their relation the help of experienced vocational teachers, reviewed by experts, tested ships.