Wildflowers of the Bitterroot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
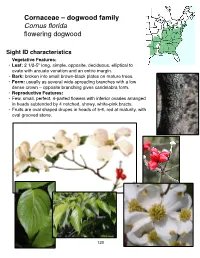
Cornaceae – Dogwood Family Cornus Florida Flowering Dogwood
Cornaceae – dogwood family Cornus florida flowering dogwood Sight ID characteristics Vegetative Features: • Leaf: 2 1/2-5" long, simple, opposite, deciduous, elliptical to ovate with arcuate venation and an entire margin. • Bark: broken into small brown-black plates on mature trees. • Form: usually as several wide-spreading branches with a low dense crown – opposite branching gives candelabra form. • Reproductive Features: • Few, small, perfect, 4-parted flowers with inferior ovaries arranged in heads subtended by 4 notched, showy, white-pink bracts. • Fruits are oval shaped drupes in heads of 5-6, red at maturity, with oval grooved stone. 123 NOTES AND SKETCHES 124 Cornaceae – dogwood family Cornus nuttallii Pacific dogwood Sight ID characteristics Vegetative Features: • Leaf: 2 1/2-4 1/2" long, simple, opposite, deciduous, ovate- elliptical with arcuate venation, margin may be sparsely toothed or entire. • Bark: dark and broken into small plates at maturity. • Form: straight trunk and narrow crown in forested conditions, many-trunked and bushy in open. • Reproductive Features: • Many yellowish-green, small, perfect, 4-parted flowers with inferior ovaries arranged in dense in heads, subtended by 4-7 showy white- pink, petal-like bracts - not notched at the apex. • Fruits are drupes in heads of 30-40, red at maturity and they have smooth stones. 125 NOTES AND SKETCHES 126 Cornaceae – dogwood family Cornus sericea red-osier dogwood Sight ID characteristics Vegetative Features: • Leaf: 2-4" long, simple, opposite, deciduous and somewhat narrow ovate-lanceolate with entire margin. • Twig: bright red, sometimes green splotched with red, white pith. • Bark: red to green with numerous lenticels; later developing larger cracks and splits and turning light brown. -

Shrub List for Brighton 2010
Shrub List For Brighton 2010 Large Shrubs 10’ -20’ Tall by 6’ – 25’ wide Acer ginnala Amur Maple Acer tataricum Tatarian Maple (better than Amur Maple) Acer grandidentatum Bigtooth Maple Amelanchier alnifolia Saskatoon Serviceberry Amelanchier canadensis Shadblow Serviceberry Caragana arborescens Siberian Peashrub Cercocarpus ledifolius Mountain Mahogany Cotoneaster lucidus Peking Cotoneaster Cowania mexicana Quince Bush, Cliffrose Crataefus ambigua Russian Hawthorn Forestiera neomexicana New Mexican Privet Hippophae rhamnoides Sea Buckthorn Juniperus species Juniper Kolkwitzia amabilis Beauty Bush Pinus mugo Mugo Pine species Prunus americana American Plum Prunus virginiana ‘Shubert’ Canada Red Chokecherry Ptelea trifoliata Wafer Ash or Hop tree Quercus gambelii Gambel Oak Rhus typhina Staghorn Sumac Robinia neomexicana New Mexico Locust Sambucus species Elders Shepherdia argentea Buffaloberry Syringa vulgaris Common Lilac Viburnum lantana Wayfaring Tree, Viburnum Medium Size Shrubs >10’ high by >8’ wide Amorpha fruticosa False Indigo Atriplex canescens Fourwing Saltbush Buddleia davidii Butterfly Bush Cercocarpus montanus Mountain Mahogany Chamaebatiaria millefolium Fernbush Chrysothamnus nauseosus Rubber Rabbitbrush Cornus sericea Redtwig Dogwood Cotinus coggygria Smoke Tree Cotoneaster species Cotoneaster Cytisus scoparius ‘Moonlight’ Moonlight Broom Euonymus alatus Burning Bush Forsythia x intermedia Forsythia Hibiscus syriacus Rose-of-Sharon Juniperus species Juniper Ligustrum vulgare Privet Lonicera species Honeysuckle Mahonia aquifolium Oregon Grape Holly Philadelphus species Mockorange Pyracantha coccinea Firethorn Physocarpus opulifolius Common Ninebark Prunus besseyi Western Sand Cherry Pyracantha coccinea species Firethorn Rhamnus frangula Glossy Buckthorn Ribes species Currant Sambucus species Elder Spiraea x vanhouttei Vanhouttei Spirea Symphoricarpos albus Snowberry Syringa meyeri „Palibin‟ Dwarf Korean Lilac Syringa patula „Miss Kim‟ Dwarf Lilac Viburnum species (dozens of different types) Small Size Shrubs > 5’ tall by >6. -

Flower Power
FLOWER POWER IDAHO BOTANICAL GARDEN WHAT IS A FLOWER? INSTRUCTIONAL OBJECTIVE: When students finish this project, they will have gained respect for the beauty of flowers and appreciate their ecological and practical importance. INTRODUCTION Dear Teacher, The Idaho Botanical Garden is an outdoor learning environment. We want to make your visit comfortable and enjoyable, and ask that your students are dressed appropriately for the weather and have water, especially in the warm weather months. TERMS Angiosperms: Flowering plants that produce seeds enclosed in a fruit. Anthers: The boxlike structures at the top of stamens, where pollen is produced. Botanical garden: A place where plants are collected and displayed for scientific, educational and artistic purposes. Fertilization: The union of male sperm cells and female egg cells. Filament: The stalk of the stamen. Flower: The reproductive structure of an angiosperm. Fruit: A ripened ovary conaining seeds. Nectar: The sweet liquid produced by flowers to attract pollinators. Ovary: The hollow compartment at the base of the pistil which contains ovules. It develops into a fruit containing seeds. Ovules: The structures in a flower ovary that can develop into seeds. Pistil: The female part of a flower; stigma, style, and ovary. Pollen: A yellow, powder-like material containing sperm cells. Pollen tubes: Tubes that carry sperm cells from the stigma into the ovary. Pollination: The process of pollen coming together with the stigma of a flower. Pollinators: Animals which carry pollen from one flower to another. Seed: A structure containing a baby plant and its food supply, which is surrounded by a protective seed coat. -

Vascular Plants at Fort Ross State Historic Park
19005 Coast Highway One, Jenner, CA 95450 ■ 707.847.3437 ■ [email protected] ■ www.fortross.org Title: Vascular Plants at Fort Ross State Historic Park Author(s): Dorothy Scherer Published by: California Native Plant Society i Source: Fort Ross Conservancy Library URL: www.fortross.org Fort Ross Conservancy (FRC) asks that you acknowledge FRC as the source of the content; if you use material from FRC online, we request that you link directly to the URL provided. If you use the content offline, we ask that you credit the source as follows: “Courtesy of Fort Ross Conservancy, www.fortross.org.” Fort Ross Conservancy, a 501(c)(3) and California State Park cooperating association, connects people to the history and beauty of Fort Ross and Salt Point State Parks. © Fort Ross Conservancy, 19005 Coast Highway One, Jenner, CA 95450, 707-847-3437 .~ ) VASCULAR PLANTS of FORT ROSS STATE HISTORIC PARK SONOMA COUNTY A PLANT COMMUNITIES PROJECT DOROTHY KING YOUNG CHAPTER CALIFORNIA NATIVE PLANT SOCIETY DOROTHY SCHERER, CHAIRPERSON DECEMBER 30, 1999 ) Vascular Plants of Fort Ross State Historic Park August 18, 2000 Family Botanical Name Common Name Plant Habitat Listed/ Community Comments Ferns & Fern Allies: Azollaceae/Mosquito Fern Azo/la filiculoides Mosquito Fern wp Blechnaceae/Deer Fern Blechnum spicant Deer Fern RV mp,sp Woodwardia fimbriata Giant Chain Fern RV wp Oennstaedtiaceae/Bracken Fern Pleridium aquilinum var. pubescens Bracken, Brake CG,CC,CF mh T Oryopteridaceae/Wood Fern Athyrium filix-femina var. cyclosorum Western lady Fern RV sp,wp Dryopteris arguta Coastal Wood Fern OS op,st Dryopteris expansa Spreading Wood Fern RV sp,wp Polystichum munitum Western Sword Fern CF mh,mp Equisetaceae/Horsetail Equisetum arvense Common Horsetail RV ds,mp Equisetum hyemale ssp.affine Common Scouring Rush RV mp,sg Equisetum laevigatum Smooth Scouring Rush mp,sg Equisetum telmateia ssp. -

Perennials for Special Purposes
Perennials for Special Purposes Hot & Dry Areas • Sage, Perennial (Artemisia) Newly planted perennials will need regular • Sea Holly (Eryngium) watering until established. • Sea Lavender (Limonium) • Spurge, Cushion (Euphorbia • Aster polychroma) • Baby’s Breath • Statice, German (Gypsophila) (Goniolimon) • Beardtongue • Stonecrop (Sedum) (Penstemon) • Sunflower, False (Heliopsis) • Big Bluestem • Sunflower, Perennial (Helianthus) (Andropogon) • Switch Grass (Panicum) • Bitterroot (Lewisia) • Tickseed (Coreopsis) • Blanketflower (Gaillardia) • Tufted Hair Grass (Deschampsia) • Blue Oat Grass (Helictotrichon) • Yarrow (Achillea) • Cactus, Prickly Pear (Opuntia) • Yucca • Candytuft (Iberis sempervirens) • Cinquefoil (Potentilla) Groundcover for Sun • Coneflower (Echinacea) • Daisy, Painted (Tanacetum) • Baby’s Breath, Creeping • Daisy, Shasta (Leucanthemum x superbum) (Gypsophila repens) • Daylily (Hemerocallis) • Beardtongue, Spreading • Evening Primrose (Oenothera) (Penstemon) • False Indigo (Baptisia) • Bellflower, Spreading • Feather Reed Grass (Calamagrostis) (Campanula) • Fescue, Blue (Festuca glauca) • Cinquefoil (Potentilla) • Flax (Linum) • Cliff Green (Paxistima • Foxtail Lily (Eremurus) canbyi) • Globe Thistle (Echinops) • Cranesbill (Geranium) • Goldenrod (Solidago) • Gentian, Trumpet (Gentiana acaulis) • Helen’s Flower (Helenium) • Globe Daisy (Globularia) • Hens & Chicks (Sempervivum) • Hens & Chicks (Sempervivum) • Ice Plant (Delosperma) • Irish/Scotch Moss (Sagina subulata) • Lamb’s Ears (Stachys byzantina) • Kinnikinnick -

Ctenophore Relationships and Their Placement As the Sister Group to All Other Animals
ARTICLES DOI: 10.1038/s41559-017-0331-3 Ctenophore relationships and their placement as the sister group to all other animals Nathan V. Whelan 1,2*, Kevin M. Kocot3, Tatiana P. Moroz4, Krishanu Mukherjee4, Peter Williams4, Gustav Paulay5, Leonid L. Moroz 4,6* and Kenneth M. Halanych 1* Ctenophora, comprising approximately 200 described species, is an important lineage for understanding metazoan evolution and is of great ecological and economic importance. Ctenophore diversity includes species with unique colloblasts used for prey capture, smooth and striated muscles, benthic and pelagic lifestyles, and locomotion with ciliated paddles or muscular propul- sion. However, the ancestral states of traits are debated and relationships among many lineages are unresolved. Here, using 27 newly sequenced ctenophore transcriptomes, publicly available data and methods to control systematic error, we establish the placement of Ctenophora as the sister group to all other animals and refine the phylogenetic relationships within ctenophores. Molecular clock analyses suggest modern ctenophore diversity originated approximately 350 million years ago ± 88 million years, conflicting with previous hypotheses, which suggest it originated approximately 65 million years ago. We recover Euplokamis dunlapae—a species with striated muscles—as the sister lineage to other sampled ctenophores. Ancestral state reconstruction shows that the most recent common ancestor of extant ctenophores was pelagic, possessed tentacles, was bio- luminescent and did not have separate sexes. Our results imply at least two transitions from a pelagic to benthic lifestyle within Ctenophora, suggesting that such transitions were more common in animal diversification than previously thought. tenophores, or comb jellies, have successfully colonized from species across most of the known phylogenetic diversity of nearly every marine environment and can be key species in Ctenophora. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Barlow Herbal Specialties Is Proud to Carry on the Legacy of Our Father, Dr
Made with Love! Medicinal Herbal Products Barlow Herbal Specialties Barlow Herbal Specialties is proud to carry on the legacy of our father, Dr. Max G. Barlow. His herbal formulas are unique, powerful and life-giving. We work from his hand-written notes to make our extracts. They are concentrated and potent. Their extraordinary strengths are achieved without heat, pressure or other mechanical means, giving you strong, high quality extracts. Our formulas are made in small batches. They are concentrated and pure. No water, preservatives, additives or fillers. Every product does what it’s designed to do. Our goal is to educate you and empower you with simple, straightforward information. To allow you to take care of your family’s everyday health challenges the way Mother Nature intended. Barlow Herbal believes that living vibrantly healthy is our right as human beings and that we must be individually responsible for our own wellness. We look forward to sharing our wonderful formulas with you and helping you sow the seeds of natural healing. www.barlowherbal.com Toll free - 866-688-6757 Local - 801-816-9241 You and your health are a vital concern to us. We sincerely hope the information we share and the products that we make help to contribute to your physical health, well-being and prosperity. Please be aware none of these statements have been evaluated by the Food and Drug Administration (FDA). These products and this information are NOT intended to diagnose, treat, cure, or prevent any disease. It is simply the 1st Amendment in action and is presented for information and research purposes only. -

Redacted for Privacy W
AN ABSTRACT OF THE THESIS OF Aaron D. Drew for the degree of Master of Science in Wildlife Science presented on December 19. 2000. Title: Effects of Livestock Grazing and Small Mammal Populations on Endangered Bradshaw's Desert Parsley (Lomatium bradshawii) at Oak Creek. Willamette Valley. Oregon. Abstract approved: Redacted for privacy W. Daniel I evaluated the response of the federally listed endangered plant species Bradshaw's desert parsley (Lomatium bradshawii) to livestock grazing and small mammal depredation at Oak Creek, Linn County, Oregon, 1997-1998. I established six study blocks (three each in wooded and herbaceous pastures) with plots in each block randomly assigned to one of four intensities of livestock grazing based on biomass remaining after grazing (no grazing [1,746 kg/ha], high biomass [969 kg/ha], moderate biomass [670 kg/ha], and light biomass [318 kg/ha]). Small mammals were live-trapped in each of the study blocks pre and post application of the livestock grazing treatments. I mapped and measured 2,807 Bradshaw's desert parsley plants (n1,366 in the wooded and n = 1,441 in the herbaceous pastures) over the two year period to determine changes in schizocarp production, morphological structure (conical surface area and height), population composition (plant stage), survival, emergence of new plants, and effects of small mammal herbivory pre and post application of livestock grazing. Grazing reductions in standing crop biomass appeared to have a positive effect on emergence of new Bradshaw's desert parsley plants, while having no detectible effect on total plant density or survival. Differences in total plant density, survival, schizocarp production, morphological structure, and population composition were related to pasture type. -

Major Lineages Within Apiaceae Subfamily Apioideae: a Comparison of Chloroplast Restriction Site and Dna Sequence Data1
American Journal of Botany 86(7): 1014±1026. 1999. MAJOR LINEAGES WITHIN APIACEAE SUBFAMILY APIOIDEAE: A COMPARISON OF CHLOROPLAST RESTRICTION SITE AND DNA SEQUENCE DATA1 GREGORY M. PLUNKETT2 AND STEPHEN R. DOWNIE Department of Plant Biology, University of Illinois, Urbana, Illinois 61801 Traditional sources of taxonomic characters in the large and taxonomically complex subfamily Apioideae (Apiaceae) have been confounding and no classi®cation system of the subfamily has been widely accepted. A restriction site analysis of the chloroplast genome from 78 representatives of Apioideae and related groups provided a data matrix of 990 variable characters (750 of which were potentially parsimony-informative). A comparison of these data to that of three recent DNA sequencing studies of Apioideae (based on ITS, rpoCl intron, and matK sequences) shows that the restriction site analysis provides 2.6± 3.6 times more variable characters for a comparable group of taxa. Moreover, levels of divergence appear to be well suited to studies at the subfamilial and tribal levels of Apiaceae. Cladistic and phenetic analyses of the restriction site data yielded trees that are visually congruent to those derived from the other recent molecular studies. On the basis of these comparisons, six lineages and one paraphyletic grade are provisionally recognized as informal groups. These groups can serve as the starting point for future, more intensive studies of the subfamily. Key words: Apiaceae; Apioideae; chloroplast genome; restriction site analysis; Umbelliferae. Apioideae are the largest and best-known subfamily of tem, and biochemical characters exhibit similarly con- Apiaceae (5 Umbelliferae) and include many familiar ed- founding parallelisms (e.g., Bell, 1971; Harborne, 1971; ible plants (e.g., carrot, parsnips, parsley, celery, fennel, Nielsen, 1971). -

Representativeness Assessment of Research Natural Areas on National Forest System Lands in Idaho
USDA United States Department of Representativeness Assessment of Agriculture Forest Service Research Natural Areas on Rocky Mountain Research Station National Forest System Lands General Technical Report RMRS-GTR-45 in Idaho March 2000 Steven K. Rust Abstract Rust, Steven K. 2000. Representativeness assessment of research natural areas on National Forest System lands in Idaho. Gen. Tech. Rep. RMRS-GTR-45. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 129 p. A representativeness assessment of National Forest System (N FS) Research Natural Areas in ldaho summarizes information on the status of the natural area network and priorities for identification of new Research Natural Areas. Natural distribution and abundance of plant associations is compared to the representation of plant associations within natural areas. Natural distribution and abundance is estimated using modeled potential natural vegetation, published classification and inventory data, and Heritage plant community element occur- rence data. Minimum criteria are applied to select only viable, high quality plant association occurrences. In assigning natural area selection priorities, decision rules are applied to encompass consideration of the adequacy and viability of representation. Selected for analysis were 1,024 plant association occurrences within 21 4 natural areas (including 115 NFS Research Natural Areas). Of the 1,566 combinations of association within ecological sections, 28 percent require additional data for further analysis; 8, 40, and 12 percent, respectively, are ranked from high to low conservation priority; 13 percent are fully represented. Patterns in natural area needs vary between ecological section. The result provides an operational prioritization of Research Natural Area needs at landscape and subregional scales. -

Identity of Mertensia Oblongifolia (Nutt.) G. Don (Boraginaceae) and Its Allies in Western North America
Great Basin Naturalist Volume 58 Number 1 Article 4 1-30-1998 Identity of Mertensia oblongifolia (Nutt.) G. Don (Boraginaceae) and its allies in western North America Ahmed M. Warfa Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Warfa, Ahmed M. (1998) "Identity of Mertensia oblongifolia (Nutt.) G. Don (Boraginaceae) and its allies in western North America," Great Basin Naturalist: Vol. 58 : No. 1 , Article 4. Available at: https://scholarsarchive.byu.edu/gbn/vol58/iss1/4 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Great Basin Naturalist 58(1), © 1998, pp. 38-44 IDENTITY OF MERTENSIA OBLONGIFOLIA (NUTT) G. DON (BORAGINACEAE) AND ITS ALLIES IN WESTERN NORTH AMERICA Ahmed M. Warfal ABSTRACT.-The current status of Mertensia oblongifoUa (Nutt.) G. Don and its allied taxa is surveyed. On the bases of continuously coherent morphological characters and/or regionally correlated variations, more than 30 taxa, including species, subspecies, varieties, and 1 forma, previously considered different from M. oblongifolia, are now placed under synonymy of this species. Those taxa currently known as M. fusiformis Greene, M. bakeri Greene, and M. bakeri var. osterhoutii Williams are among the new synonyms. Typification, taxonomy, and morphological problems ofM. oblongifo lia are discussed. Key words: Mertensia oblongifolia, typification, Wxorwmy, morphology, allied taxa. Nuttall (1834) described and depicted Pul represents an important additional morpholog monaria oblongifolia from a collection ofplants ical feature in the taxon.