MAVURU ALLEN (B1128943)ES.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

African Development Bank African Development Fund
AFRICAN DEVELOPMENT BANK AFRICAN DEVELOPMENT FUND Public Disclosure Authorized Disclosure Public BULAWAYO WATER AND SEWERAGE SERVICES IMPROVEMENT PROJECT COUNTRY: ZIMBABWE APPRAISAL REPORT Public Disclosure Authorized Disclosure Public OWAS DEPARTMENT November 2015 TABLE OF CONTENTS SUMMARY ................................................................................................................................................ iii RESULTS- BASED LOGICAL FRAMEWORK ................................................................................... iv I – STRATEGIC THRUST & RATIONALE .............................................................................................. 1 1.1 Project Linkages with Country Strategy and Objectives ............................................................ 1 1.2 Rationale for Bank’s Involvement .............................................................................................. 2 1.3 Donor Coordination .................................................................................................................... 3 II. PROJECT DESCRIPTION ...................................................................................................................... 4 2.1 Detailed Project Components ...................................................................................................... 4 2.2 Technical Solution Retained and Other Alternatives Explored .................................................. 5 2.3 Project Type ............................................................................................................................... -

PLAAS RR46 Smeadzim 1.Pdf
Chrispen Sukume, Blasio Mavedzenge, Felix Murimbarima and Ian Scoones Faculty of Economic and Management Sciences Research Report 46 Space, Markets and Employment in Agricultural Development: Zimbabwe Country Report Chrispen Sukume, Blasio Mavedzenge, Felix Murimbarima and Ian Scoones Published by the Institute for Poverty, Land and Agrarian Studies, Faculty of Economic and Management Sciences, University of the Western Cape, Private Bag X17, Bellville 7535, Cape Town, South Africa Tel: +27 21 959 3733 Fax: +27 21 959 3732 Email: [email protected] Institute for Poverty, Land and Agrarian Studies Research Report no. 46 June 2015 All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior permission from the publisher or the authors. Copy Editor: Vaun Cornell Series Editor: Rebecca Pointer Photographs: Pamela Ngwenya Typeset in Frutiger Thanks to the UK’s Department for International Development (DfID) and the Economic and Social Research Council’s (ESRC) Growth Research Programme Contents List of tables ................................................................................................................ ii List of figures .............................................................................................................. iii Acronyms and abbreviations ...................................................................................... v 1 Introduction ........................................................................................................ -

Promotion of Climate-Resilient Lifestyles Among Rural Families in Gutu
Promotion of climate-resilient lifestyles among rural families in Gutu (Masvingo Province), Mutasa (Manicaland Province) and Shamva (Mashonaland Central Province) Districts | Zimbabwe Sahara and Sahel Observatory 26 November 2019 Promotion of climate-resilient lifestyles among rural families in Gutu Project/Programme title: (Masvingo Province), Mutasa (Manicaland Province) and Shamva (Mashonaland Central Province) Districts Country(ies): Zimbabwe National Designated Climate Change Management Department, Ministry of Authority(ies) (NDA): Environment, Water and Climate Development Aid from People to People in Zimbabwe (DAPP Executing Entities: Zimbabwe) Accredited Entity(ies) (AE): Sahara and Sahel Observatory Date of first submission/ 7/19/2019 V.1 version number: Date of current submission/ 11/26/2019 V.2 version number A. Project / Programme Information (max. 1 page) ☒ Project ☒ Public sector A.2. Public or A.1. Project or programme A.3 RFP Not applicable private sector ☐ Programme ☐ Private sector Mitigation: Reduced emissions from: ☐ Energy access and power generation: 0% ☐ Low emission transport: 0% ☐ Buildings, cities and industries and appliances: 0% A.4. Indicate the result ☒ Forestry and land use: 25% areas for the project/programme Adaptation: Increased resilience of: ☒ Most vulnerable people and communities: 25% ☒ Health and well-being, and food and water security: 25% ☐ Infrastructure and built environment: 0% ☒ Ecosystem and ecosystem services: 25% A.5.1. Estimated mitigation impact 399,223 tCO2eq (tCO2eq over project lifespan) A.5.2. Estimated adaptation impact 12,000 direct beneficiaries (number of direct beneficiaries) A.5. Impact potential A.5.3. Estimated adaptation impact 40,000 indirect beneficiaries (number of indirect beneficiaries) A.5.4. Estimated adaptation impact 0.28% of the country’s total population (% of total population) A.6. -

Covid-19 Addendum
ZIMBABWE COVID-19 ADDENDUM Part 6 COVID-19 Addendum SHAMVA DISTRICT, MASHONALAND CENTRAL PROVINCE A beneficiary washes her hands before entering a food distribution in Shamva district. Photo:WFP/Claire Nevill 46 ZIMBABWE COVID-19 ADDENDUM COVID-19 Response at a Glance PEOPLE IN NEED PEOPLE TARGETED REQUIREMENTS (US$) OPERATIONAL PARTNERS 7.5M 5.9M $84.9M 37 People in Need and Targeted Requirements by Cluster H M Me Hurungwe Cy/ Mabani M F S M U Dwin Rushinga MASHONALAND CENTRAL Karoi M Guruve Mwi M Shamva M AIBA Kariba Zvimba B Pfungwe Cyi U M Mowe MASHONALAND EAST Education Ba Moko Nth MASHONALAND WEST Nton Goromonzi HARARE Mehwa B South Sanyati C Water S U Nanga V Monder M Kadoma M Town Seke U U C H WASH U MIDLANDS Mondera MANICALAND M-N Hdweza Rusape M Lupane Kwekwe MATEBELELAND NORTH K Nyi C N U M Hwange Redcliff M U C Ba urban Gutu Me Tsholotsho B Gweru U U P BV Byo C M MASVINGO I B C M Zvishavane U B Zvishavane U Z U U M C Potection Plumtree Mengwa MATEBELELAND SOUTH 2M C C Potection U U M Mobo M Gwanda Cedzi B SM M Propor IN Targeted B P geted U R 02M People in Need and Targeted by Cluster Operational Partners by Type Water S M H WASH 2M F S 0M M NNO Education M 9 M INO H M 18 0M PBV 2M 845K PP 22M UN 22 0 MS 20M 20 0M N 353K 2 S 91K In addition to the humanitarian response requirements, R 14K $4.5 million is needed to support Governance interventions 14K dination and $22.5 million for social protection, which will be 953 M 953 carried out by development actors. -

National Rapid Response Team Contacts
National Rapid Response Team Contacts City/Town Contact Person Mobile Number Toll free Number Institution/Role Ace Ambulance +263 782999901-4 (0) 8080412 Harare ZRP (0242) 777777 ZRP Harare - Wilkins (0242) 741872 Wilkins Hosp Harare - Wilkins (0242) 740404 Wilkins Hosp Harare Dr Chonzi +263 712860777 Harare Dr Bara +263 734322293 Harare Dr Mudariki +263 772974314 Bulawayo Ms Sibanda +263 772677476 Bulawayo Dr Nyathi +263 776248128 Bulawayo Dr Ncube +263 772424812 Bulawayo Dr E Sibanda +263 772880581 Director Health Services Bulilima Dr Hapanyengwi +263 772907621 Beitbridge Dr Samhere +263 772386895 Bindura Mr Karisa +263 773271670 DMO Bikita Dr Mungwari +263 715411650 Centenary/Muzarabani Mr Kangundu +263 777366045 DNO Chegutu Dr Masvosva +263 772720190 Chiredzi Dr Dhlandhlara +263 775094360 Chirumanzu Dr S Maunga +263 772286685 (0) 8080435 Chirumanzu Mr Mukomberanwa +263 773 394 154 (0) 8080435 Chirumanzu Sr Mutumwa +263 772 911 454 (0) 8080435 Concession Dr Sosera +263 774736753 Gokwe North DR Chikara +263 775 428800 Gokwe North E Muchenje +263772 575437 City/Town Contact Person Mobile Number Toll free Number Institution/Role Gokwe South DR Mashoko +263 774 074739 Gokwe South D Mukotsi +263 774 002 934 Goromonzi Dr Karim +263 772347378 Guruve Zvomuya +263 772641444 DNO Gwanda Dr Gwarimbo +263 775735679 Gweru Dr Mhene +263 773258210 (0) 8080435 Gweru Provincial Hosp Toll free +263 787822276 (0) 8080435 Gweru Provincial Hospital Gweru& City G Shariwa +263 773 639 797 (0) 8080435 Gweru& City Mr Sekanhamo +263 715017014 (0) 8080435 Gweru& -

The Mineral Industry of Zimbabwe in 2010
2010 Minerals Yearbook ZIMBABWE U.S. Department of the Interior August 2012 U.S. Geological Survey THE MINERAL INDUSTRY OF ZIMBABWE By Philip M. Mobbs Zimbabwe’s diverse mineral output included about 5% of the 2010 Mid Term Fiscal Policy Statement. Royalties on the world’s platinum production and about 3% of the world’s base metals remained at 2% of gross revenue (Central African palladium output. In 2010, additional mineral production Gold PLC, 2010, p. 3; Kramarenko and others, 2010, p. 39). companies resumed operations at mines and plants that had been temporarily suspended (placed on care-and-maintenance status) Production in the past few years. Few companies, however, were operating at full capacity owing in part to the difficulty in securing In 2010, production of numerous mineral commodities funding and the ongoing uncertainty concerning the pace of increased significantly owing to the Government’s changes the indigenization process in the mining sector (which would in legislative and monetary policies in 2009. Such changes affect future equity ownership of mineral operations) (table 1; included the temporary replacement of the Zimbabwe dollar International Monetary Fund, 2011, p. 21–23; Loferski, 2011). by a multicurrency national financial system, which aided In 2010, the real gross domestic product growth was the industry’s recovery from hyperinflation that had ravaged estimated to be 9% compared with 6% in 2009 and –18% in Zimbabwe’s economy in 2008 and early 2009, and allowing 2008. The inflation rate was down to an estimated 3% in 2010 organizations to export gold instead of selling it to the compared with 6.5% in 2009 and an estimated average of Government. -

Health Cluster Bulletin 11Ver2
Zimbabwe Health Cluster bulletin Bulletin No 11 1-15 April 2009 Highlights: Cholera outbreak situation update • About 96, 473 cases and 4,204 deaths, CFR 4.4% Following a 9 week decline trend in cholera cases, an upsurge was reported during epidemi- • Sustained decline of ological week 15. Batch reporting in three districts may have contributed to this slight in- the outbreak crease. • Cholera hotspots in The cumulative number of Mashonaland west, Cholera in Zimbabwe reported cholera cases was Harare and Chitungwiza 17 Aug 08 to 11th April 09 96, 473 and 4204 deaths with 10,000 cities cumulative Case Fatality Rate 8,000 Cases Deaths (CFR) as of 4.4 as of 15 April. During week 15, a 17% de- 6,000 crease in cases and 5% in- 4,000 crease in deaths was re- Number ported. The crude CFR is 2.7% 2,000 compared to 2.9% of week 14 0 while the I-CFR is 1.8% com- pared to 2.7% of week 14. The w2 w4 w6 w8 w36 w38 w40 w42 w44 w46 w48 w50 w52 w10 w12 w14 CFR has been steadily de- weeks clined although the proportion of deaths in health facilities has increased compared to Cholera in Zimbabw e from 16 Nov 08 to 11th A pril 09 those reported in the commu- W eekly c rude and institutional c ase-fatality ratios 10 nity. CFR 9 This is probably an indication Inside this issue: 8 iCFR 7 of more people accessing 6 treatment and/or the increas- Cholera situation 1 5 ing role of other co- 4 morbidities presenting along- ORPs in cholera 2 3 management percent side cholera. -
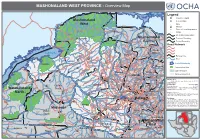
MASHONALAND WEST PROVINCE - Overview Map
MASHONALAND WEST PROVINCE - Overview Map Kanyemba Mana Lake C. Bassa Pools Legend Province Capital Mashonaland Key Location r ive R Mine zi West Hunyani e Paul V Casembi b Chikafa Chidodo Mission Chirundu m Angwa Muzeza a Bridge Z Musengezi Place of Local Importance Rukomechi Masoka Mushumbi Musengezi Mbire Pools Chadereka Village Marongora St. International Boundary Cecelia Makuti Mashonaland Province Boundary Hurungwe Hoya Kaitano Kamuchikukundu Bwazi Chitindiwa Muzarabani District Boundary Shamrocke Bakasa Central St. St. Vuti Alberts Alberts Nembire KARIBA Kachuta Kazunga Chawarura Road Network Charara Lynx Centenary Dotito Kapiri Mwami Guruve Mount Lake Kariba Dora Shinje Masanga Centenary Darwin Doma Mount Maumbe Guruve Gachegache Darwin Railway Line Chalala Tashinga KAROI Kareshi Magunje Bumi Mudindo Bure River Hills Charles Mhangura Nyamhunga Clack Madadzi Goora Mola Mhangura Madombwe Chanetsa Norah Silverside Mutepatepa Bradley Zvipane Chivakanyama Madziwa Lake/Waterbody Kariba Nyakudya Institute Raffingora Jester Mvurwi Vanad Mujere Kapfunde Mudzumu Nzvimbo Shamva Conservation Area Kapfunde Feock Kasimbwi Madziwa Tengwe Siyakobvu Chidamoyo Muswewenhede Chakonda Msapakaruma Chimusimbe Mutorashanga Howard Other Province Negande Chidamoyo Nyota Zave Institute Zvimba Muriel Bindura Siantula Lions Freda & Mashonaland West Den Caesar Rebecca Rukara Mazowe Shamva Marere Shackleton Trojan Shamva Chete CHINHOYI Sutton Amandas Glendale Alaska Alaska BINDURA Banket Muonwe Map Doc Name: Springbok Great Concession Manhenga Tchoda Golden -

ZIMBABWE - Transportation Infrastructure
ZIMBABWE - Transportation Infrastructure Mukumbura Legend MOZAMBIQUE Populated Places National Capital Chirundu Province Capital Port of Entry Mbire ZAMBIA Transportation Hurungwe Aerodrome Kariba Centenary Mount Airport Rushinga Guruve Darwin Road network Mashonaland Nyamapanda Primary Kariba Railway Line Central Mudzi Mashonaland Shamva Admin Boundaries Mazowe BINDURA International Boundary West CHINHOYI Bindura Makonde Mutoko Province Boundary Gokwe District Boundary North Zvimba Murehwa Kazungula Victoria Mashonaland NAMIBIA Binga Lake Kariba Falls Harare East Goromonzi Sanyati MARONDERA Conservation Area Gokwe Chegutu Seke Nyanga South Marondera Mhondoro- Makoni Hwange Ngezi Hwedza Mutasa Matabeleland Kwekwe Pandamatenga LUPANE Nkayi Chikomba Manicaland North Forbes Lupane MUTARE Map Doc Name: ZWE003_Zimbabwe_Trans Infrastructure_A4_05102009 GLIDE Number: Midlands Mutare Chirumhanzu Creation Date: 5 October 2009 GWERU Projection/Datum: Arc 1950 Buhera Gweru Tsholotsho Web Resources: http://ochaonline.un.org/zimbabwe 1:4,554,309 Bubi Gutu Nominal Scale at A4 paper size: Shurugwi Chimanimani 0 50 100 150 kms Umguza MASVINGO BULAWAYO Masvingo Map data source(s): Bulilima Insiza Bikita Vector data from Department of the Surveyor General Bulawayo (DSG) and Central Statistical Office (CSO). Umzingwane Zvishavane Chipinge Mt Selinda Plumtree Zaka Disclaimers: Chivi The designations employed and the presentation of Masvingo material on this map do not imply the expression of any Matobo opinion whatsoever on the part of the Secretariat of the Mberengwa United Nations concerning the legal status of any Mangwe country, territory, city or area or of its authorities, or Matabeleland concerning the delimitation of its frontiers or boundaries. BOTSWANA South Gwanda Mwenezi E Chiredzi U IQ B M Beitbridge A Sango Z Beitbridge O M SOUTH AFRICA. -

MASVINGO PROVINCE - Basemap
MASVINGO PROVINCE - Basemap Mashonaland East Mpinda Musuma Msilahove Ntabamhlope Ntabamhlope 30 Hunters Mlezu 19 27 Chikwava 8 Munyanyi Chipfatsura Mushunje Rowa 6 C h i c a m b a e a l 3 Road 15 31 4 33 R 25 23 12 Lancashire Chipwanya 1 31 13 Gunde Chapwanya 10 15 Burma Madilisa Nkululeko Connemara Murezi Mombeyarara 4 Gunde Central Garamwera Bepe 2 11 Valley Locations Nyama Masvori Chirinda 12 14 Connemara St Patricks Estates 8 14 CHIKOMBA 2 11 Chiweshe Zumbare KWEKWE 7 12 7 Nyama 12 Gombe 13 Chiwenga 9 Berzerly 9 St. Muchakata 13 Maburutse 3 Matanda Bridge 11 St. Gwindingwi 10 3 Patricks Chiundura 5 Makumbe Richards Marange Madhikani 16 Murambinda Nyashanu Bazeley Province Capital 2 Maboleni Marange 24 7 Mvuma 24 Nyashanu Murambinda Nyashanu 18 Bridge 20 Mambwere Maboleni 2 Nerutanga 10 Mvuma 13 Denhere 8 O Cambrai 20 Bakorenhema Athens d Netherburn N y a m a f u f u 36 21 4 z Broadside Buhera Nhamo i Chitora Chipendeke s 15 Lalapanzi d 22 Buhera 18 Nyangani Zvipiripiri 17 Chikwariro 19 Chitora BANTI n 6 Lalapanzi 6 14 Town a 17 Mtao Bambazonke l W h i t e w a t e r Whahwa 16 Bwizi O 5 1 Mudanda 20 Nzvenga d d Fairfield z i Gutaurare Lower i Hlabano Mangwande Insukamini Dambara Betera 26 Mpudzi M Gweru 8 16 14 1 I n s u k a m i n i BUHERA MUTARE Muromo 19 4 Mudanda 22 Makepesi O M Vungu Sino Rukundo 11 Lynwood Driefontein Nyazvidzi Viriri 17 23 Place of Local Importance Lower 25 d Zimbabwe Driefontein St Andrews z 27 a Gweru Welcome i n Totonga Isolation 1 Nyazvidzi i Muwonde Felixburg 16 Madzimbashuro Zvipiripiri Masasi c Soti a Mkoba -

Marasha-Tapesana.Pdf
BINDURA UNIVERSITY OF SCIENCE EDUCATION FACULTY OF EDUCATION DEPARTMENT OF MATHS & PHYSICS AN ASSESSMENT OF MISCONCEPTIONS IN THE USE OF SOLAR CELLS AS SOURCES OF ELECTRICITY. A CASE OF GUTU HIGH SCHOOL ‘A’ LEVEL STUDENTS BY MARASHA TAPESANA B1544992 SUPERVISOR: MR NDLOVU A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR AN HONOURS BACHELOR OF SCIENCE DEGREE IN PHYSICS. (BINDURA UNIVERSITY OF SCIENCE EDUCATION) SEPTEMBER 2018 APPROVAL FORM The undersigned certify that they have supervised the Student research project entitled, An assessment of misconceptions in the use of solar cells as sources of electricity; A case of Gutu High School A Level students. Submitted in Partial fulfilment of the requirements of HONOURS BACHELOR OF SCIENCE DEGREE IN PHYSICS at Bindura University of Science Education. ………………………………/…………… …………/………………….. Student Signature Date ………………………………/…………… …………/………………….. Supervisor Signature Date …….…………………………/…………… …………/………………….. Chairperson Signature Date ………………………………/…………… …………/…………………. External Examiner Signature Date RELEASE FORM i Name of Student : MARASHA TAPESANA Reg number : B1544992 Dissertation Title : AN ASSESSMENT OF MISCONCEPTIONS IN THE USE OF SOLAR CELLS: A CASE OF GUTU HIGH STUDENTS Degree Title : HONOURS BACHELOR OF SCIENCE DEGREE IN PHYSICS Year This Degree Granted : 2018 Permanent Address : Gutu High School Bag 901 Gutu Cell: 0775 301 357 Permission is hereby granted to Bindura University of Science Education Library to produce single copies of this dissertation and to lend or sell such copies for private, scholarly or scientific research purpose only. The author reserves other publication rights and no extensive extracts from it may be printed or reproduced without the author’s written permission. SIGNED.......................................... DECLARATION ii I, MARASHA TAPESANA declare that: this is my original work and it had not been submitted to this or any other University. -

'O' Level Final Round Results To
BINDURA UNIVERSITY OF SCIENCE EDUCATION FACULTY OF SCIENCE SCIENCE AND MATHEMATICS OLYMPIAD ‘O’ LEVEL FINAL ROUND RESULTS TOP PERFORMERS BY SUBJECT (‘O’ LEVEL) AT NATIONAL LEVEL 1.1 ‘O’ LEVEL BIOLOGY TOP FIVE PERFORMERS 2018 SN NAME OF CANDIDATE SCHOOL PROVINCE SCORE RANK 1 GANYAUPFU VALENTINE HANKE ADVENTIST MIDLANDS 82 1 2 HARI BRAINDON ST MARY'S HIGH HARARE 78 2 3 RUWENDE VENGERE HIGH MANICALAND 76 3 ZVIKOMBORERO 4 CHIRIVAMBERI RICHWELL SAINT FAITH'S MANICALAND 75 4 5 MUTORE NICKSENSE QUEEN ELIZABETH HARARE 74 5 1.2 ‘O’ LEVEL CHEMISTRY TOP FIVE PERFORMERS 2018 SN NAME OF CANDIDATE PROVINCE SCHOOL SCORE RANK 1 TANDE SHINGIRAI HARARE ZENGEZA 82 1 2 HUKUIMWE JOSHUA MANICALNAD MARIST 81 2 NYANGA 3 NDLOVU KELVIN S. MIDLANDS NJELELE HIGH 80 3 4 RICHWELL CHIRIPAMBERI MASH WEST ST. FRANCIS 79 4 5 DENDERA T. ADMIRE MASH CENTRAL ST ALBERT’S 78 5 1.3 ‘O’ LEVEL COMPUTER SCIENCE TOP FIVE PERFORMERS 2018 SN SURNAME NAME SCHOOL PROVINCE SCORE RANK 1 DAMBAKUSHAMBA KAREN KRISTE MAMBO MANICALAND 81 1 2 MUSENDO MARTHA KRISTE MAMBO MANICALAND 74 2 3 MUKOGO MALVERN ST FAITH HIGH MANICALAND 72 3 4 MUTEVEDZI ANDIPANYASHA ZRP HIGH HARARE 70 4 5 NGWENYA CHIEDZA SILVEIRA HIGH MASVINGO 69 5 Page 1 of 20 1.4 ‘O’ LEVEL GEOGRAPHY TOP FIVE PERFORMERS 2018 SN NAME OF CANDIDATE SCHOOL PROVINCE SCORE RANK 1 GANYAUPFU VALENTINE HANKE HIGH MIDLANDS 90 1 2 MUKOGO MALVERN SAINT FAITH'S HIGH MANICALAND 88 2 3 MHINDU MIAN HANKE HIGH MIDLANDS 84 3 4 MTANDAGARI POWENETTY KWEKWE HIGH MIDLANDS 82 4 5 MHLANGA RACHEAL CHAPLIN HIGH MIDLANDS 82 4 1.5 ‘O’ LEVEL MATHEMATICS TOP FIVE PERFORMERS 2018 SN NAME SURNAME SCHOOL PROVINCE SCORE RANK 1 TAFADZWA ZINZOU ZRP HIGH SCHOOL HARARE 93 1 2 NYARUWAKA JEMIMA KNOWSTICS MANICALAND 89 2 ACADEMY 3 JOYCE MUNHENGA MANDEDZA HIGH MASH.