Quantitative Determination of Norethisterone Acetate in Human Plasma by Capillary Gas Chromatography with Mass-Selective Detection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anabolic-Androgenic Steroids in Horses: Natural Presence and Underlying Biomechanisms
ANABOLIC-ANDROGENIC STEROIDS IN HORSES: NATURAL PRESENCE AND UNDERLYING BIOMECHANISMS Anneleen Decloedt Dissertation submitted in the fulfilment of the requirements for the degree of Doctor of philosophy (PhD) in Veterinary Sciences, Faculty of Veterinary Medicine, Ghent University PROMOTER Prof. dr. ir. Lynn Vanhaecke Ghent University, Faculty of Veterinary Medicine Department of Veterinary Public Health and Food Safety Laboratory of Chemical Analysis MEMBERS OF THE READING COMMITTEE Prof. dr. James Scarth HFL Sport Science, Cambridgeshire, United-Kingdom Prof. dr. Peter Van Eenoo Ghent University, DoCoLab, Zwijnaarde, Belgium Prof. dr. Ann Van Soom Ghent University, Faculty of Veterinary Medicine, Merelbeke, Belgium MEMBERS OF THE EXAMINATION COMMITTEE Dr. Ludovic Bailly-Chouriberry Laboratoires des Courses Hippiques, Verrières-le-Buisson, France Dr. Leen Van Ginkel Wageningen University, RIKILT, Wageningen, The Netherlands Prof. dr. Myriam Hesta Ghent University, Faculty of Veterinary Medicine, Merelbeke, Belgium This work was funded by the Fédération Nationale des Courses Françaises (via the Laboratoire des Courses Hippiques) and executed at the Laboratory of Chemical Analysis (Faculty of Veterinary Medicine, Ghent University, Merelbeke). The author and the promoter give the authorisation to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. “The universe is full of magic, Just patiently waiting for our wits to grow sharper” TABLE OF CONTENTS TABLE OF CONTENTS Chapter I – General Introduction 1 1. Steroids 3 1.1 Chemical structure 1.2 (Steroid) hormones and their role in the endocrine system 1.3 Biosynthesis of steroid hormones 1.4 Anabolic-androgenic steroids (AAS) 1.5 Synthesis and absorption of the steroid precursor cholesterol 2. -

Quick Determination of 105 Doping in Animal Derived Food By
QTRAP®质谱系统对动物源食品中105种兴奋剂的快速分析 检测 Quick Determination of 105 Doping in Animal – derived Food by QTRAP® System 杨总1,陈丹2,程海燕1,李立军1,郭立海1 Yang Zong1,Chen Dan2,Cheng Haiyan1,Li Lijun1,Guo Lihai1 1 SCIEX亚太应用支持中心,上海; 2 湖北省武汉食品化妆品检验所 1 SCIEX Asia Pacific Application Support Center, Shanghai, China; 2 Wuhan Institute for Food and Cosmetic Control, Hubei Province, China; Keywords:doping;SCIEX QTRAP® 4500 系统; LC-MS/ MS;quantitative analysis 引言 在现代畜牧养殖业中,一些不法分子长期使用各种饲料添加 剂和人工合成激素类化合物,造成动物源食品中的药物残留,最 终成为食源性兴奋剂的来源[1]。运动员食用含有某些药物残留的食 物,可导致其兴奋剂检测呈阳性。国家反兴奋剂中心和世界反兴 奋剂机构(WADA)等组织机构明确规定了兴奋剂的违禁药物的种 SCIEX ExionLC™液相和SCIEX QTRAP® 4500质谱系统 类,主要包含了β-受体激动剂、固醇类激素、糖皮质激素、利尿 剂、玉米赤霉醇类等等。总体而言,兴奋剂的作用主要是对身体或 者中枢神经系统起到选择性的作用,能促进肌肉组织快速增长、增 4. 建立好了105种兴奋剂EPI二级谱库,软件自动进行匹配二级质 强机体活力和敏捷度,还可使运动员疲劳减轻,自信心增强,注意 谱图,保证筛查结果准确可靠; 力集中[2]。因此在体育赛事过程中,为了保证赛事结果的公平、公 正,在运动员食品安全保障过程中,必须对食品中兴奋剂进行严格 5. 方法现成,省去开发方法的时间,提高了工作效率; 的监控。本文针对动物源食品中的常见105种兴奋剂,建立了采用 QTRAP®系统快速筛查和定量方法,该方法完全满足在体育赛事过 1. 实验方法 程中对兴奋剂监管的分析要求。 1.1 液相色谱条件 该方案的特点和优势 色谱柱:Phenomenex Kinetex 2.6µm F5 100 Å 150×3 µm 流动相:水相(水中含有5 mM乙酸铵和0.01%甲酸),有机 1. 方法覆盖面广,包含了常见五大类β-受体激动剂、固醇类激 相为乙腈,梯度洗脱 素、糖皮质激素、利尿剂、玉米赤霉醇类兴奋剂,共105种; 1.2 质谱条件 2. 本实验详细优化了前处理过程,考察了化合物的稳定性; 扫描模式:MRM-IDA-EPI,正负离子同时扫描,MRM离子对见 3. 实验采用MRM-IDA-EPI扫描实现一针进样同时定量和定性分 表1。 析,快速灵敏,满足国内外标准品的要求; RUO-MKT-02-9756-ZH-A p 1 离子源:ESI源;离子源参数:喷雾电压(IS):5500V(+) 2. 结果与讨论 /-4500V(-);离子源温度:600℃;气帘气(CUR):35psi;碰 2.1 前处理过程的优化 撞气(CAD):Medium;雾化气(GS1):55psi;辅助雾化气 (GS2):60psi 本实验详细优化了前处理过程,实验结果表明在动物源食品 中酸化乙腈的提取效率最高,绝大部分化合物回收率在70%以上 1.3 样品前处理过程 (见图1、图2、图3)。由于基质的复杂性,实验比较了5种不同 称取5g样品于50 mL离心管 → 加入10 mL乙酸铵缓冲溶液混 的SPE柱的净化效率,由图4可以看出,PRIME HLB 净化效果最优 异,回收率较高。 匀 → 加入β-葡萄糖醛甙酶进行酶解 → 加入酸化乙腈进行提取, 震荡混匀后加入NaCl和Mg2SO4 → 萃取之后高速离心取上清液采用 PRIME HLB进行净化 → 吹干后复溶上机测试。 100 80 60 40 20 0 酸化叔丁基甲醚 酸化乙腈 碱化乙腈 图1. -

(12) Patent Application Publication (10) Pub. No.: US 2008/0063698 A1 Hartwig (43) Pub
US 2008 OO63698A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0063698 A1 Hartwig (43) Pub. Date: Mar. 13, 2008 (54) CRYSTALLIZATION INHIBITION OF DRUGS now abandoned, which is a continuation of applica IN TRANSIDERMAL DRUG DELIVERY tion No. 10/010,342, filed on Dec. 5, 2001, now SYSTEMIS AND METHODS OF USE abandoned. (75) Inventor: Rod Lawson Hartwig, Plantation, FL (60) Provisional application No. 60/251.294, filed on Dec. (US) 5, 2000. Correspondence Address: Publication Classification Noven Pharmaceuticals, Inc. Jay G. Kolman, Esq. (51) Int. Cl. 11960 S.W. 144 Street A6II 3/56 (2006.01) A6F 3/00 (2006.01) Miami, FL 33186 (US) A6IP 43/00 (2006.01) (73) Assignee: NOVEN PHARMACEUTICALS, A6F 3/02 (2006.01) INC. (52) U.S. Cl. ........................... 424/448; 424/449; 514/170 (57) ABSTRACT Appl. No.: 11/897,189 (21) The invention relates to compositions and methods for (22) Filed: Aug. 29, 2007 making a transdermal drug delivery system capable of achieving substantially zero-order kinetics for delivery of Related U.S. Application Data the active agent over a period of time in excess of 24 hours and at least 72 hours, comprising a pharmaceutically accept (63) Continuation of application No. 10/751,152, filed on able active agent carrier and a rosin ester which provides a Jan. 2, 2004, now abandoned, which is a continuation crystal inhibiting and drug stabilizing effect on the active of application No. 10/353,624, filed on Jan. 29, 2003, agents incorporated therein. 13 2 2 Patent Application Publication Mar. -

Control Be Implemented. the First Large-Scale Systematic Olympic Games
Br J Sports Med: first published as 10.1136/bjsm.11.4.162 on 1 December 1977. Downloaded from 162 Brit. J. Sports Med. - Vol. 1 1, No. 4, December 1977, pp. 162-169 RADIOIMMUNOASSAY OF ANABOLIC STEROIDS: AN EVALUATION OF THREE ANTISERA FOR THE DETECTION OF ANABOLIC STEROIDS IN BIOLOGICAL FLUIDS Robert DUGAL, Ph.D., Claire DUPUIS, Ph.D. and Michel J. BERTRAND, Ph.D. Centre de Recherches en Sciences de la Sante, Institut National de la Recherche Scientifique, Centre Hospitalier Louis-H. Lafontaine, Montrdal, Quebec, Canada ABSTRACT Recently developped radioimmunoassays (RIA) for the analysis of anabolic steroids and their metabolites in biological fluids were tested for cross-reactivity with other types of steroids. Results show that the degree of desirable cross-reactivity within the two classes of orally active anabolic steroids vary widely and that the antiserum for 19-Nortestosterone (the active principle of intramuscular, preparations) has a very high degree of undesirable cross- reactivity with components of oral contraceptives. Single and multiple dose studies in human volunteers demonstrate that the detection level and degree of retrospectivity are likewise variable but that the test easily detects most anabolic steroids during treatment. At the present time, the combination of the three antisera for the assay of a sample appears to be a relatively rapid and economic method for screening large numbers of samples in situations where doping control of anabolic steroids is required. The importance of utilizing physico-chemical means for identification of RIA potential positives is emphasized. INTRODUCTION (Sumner, 1974: Brooks et al, 1975) and identification procedures for a limited number of them have also been copyright. -

Nomenclature of Steroids
Pure&App/. Chern.,Vol. 61, No. 10, pp. 1783-1822,1989. Printed in Great Britain. @ 1989 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY and INTERNATIONAL UNION OF BIOCHEMISTRY JOINT COMMISSION ON BIOCHEMICAL NOMENCLATURE* NOMENCLATURE OF STEROIDS (Recommendations 1989) Prepared for publication by G. P. MOSS Queen Mary College, Mile End Road, London El 4NS, UK *Membership of the Commission (JCBN) during 1987-89 is as follows: Chairman: J. F. G. Vliegenthart (Netherlands); Secretary: A. Cornish-Bowden (UK); Members: J. R. Bull (RSA); M. A. Chester (Sweden); C. LiCbecq (Belgium, representing the IUB Committee of Editors of Biochemical Journals); J. Reedijk (Netherlands); P. Venetianer (Hungary); Associate Members: G. P. Moss (UK); J. C. Rigg (Netherlands). Additional contributors to the formulation of these recommendations: Nomenclature Committee of ZUB(NC-ZUB) (those additional to JCBN): H. Bielka (GDR); C. R. Cantor (USA); H. B. F. Dixon (UK); P. Karlson (FRG); K. L. Loening (USA); W. Saenger (FRG); N. Sharon (Israel); E. J. van Lenten (USA); S. F. Velick (USA); E. C. Webb (Australia). Membership of Expert Panel: P. Karlson (FRG, Convener); J. R. Bull (RSA); K. Engel (FRG); J. Fried (USA); H. W. Kircher (USA); K. L. Loening (USA); G. P. Moss (UK); G. Popjiik (USA); M. R. Uskokovic (USA). Correspondence on these recommendations should be addressed to Dr. G. P. Moss at the above address or to any member of the Commission. Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with IUPAC copyright symbol (01989 IUPAC), is printed. -

Regioselective Enzymatic Acylation of Vicinal Diols of Steroids
Tetrahedron 61 (2005) 3065–3073 Regioselective enzymatic acylation of vicinal diols of steroids M. Manuel Cruz Silva,a Sergio Rivab and M. Luisa Sa´ e Meloa,* aCentro de Estudos Farmaceˆuticos, Lab. Quı´mica Farmaceˆutica, Faculdade de Farma´cia, Universidade de Coimbra, Rua do Norte 3000-295 Coimbra, Portugal bIstituto di Chimica del Riconoscimento Molecolare, C.N.R., Via Mario Bianco 9, 20131 Milano, Italy Received 28 October 2004; revised 29 November 2004; accepted 25 January 2005 Abstract—Monoacylated derivatives of a complete set of 2,3- and 3,4-vicinal diols of steroids were prepared by regioselective lipase- catalysed transesterification reactions. The enzymes displayed different selectivities towards the vicinal diols depending on the configuration of the hydroxyl groups. q 2005 Elsevier Ltd. All rights reserved. 1. Introduction Studies on the transesterification of polyfunctionalyzed steroids have shown that hydrolases can have access to Polyhydroxylated steroids bearing vicinal diols on the substituents either on the A-ring or on the D-ring and/or on A-ring are frequently found in Nature and some of them the side-chain of steroids. Several lipases showed a have relevant biological activities. For instance, the trans- preference for C-3 hydroxyl groups,13,14 whereas the diaxial 2b,3a-di-hydroxy pattern is present in natural protease subtilisin Carlsberg catalysed the acylation of 14 sulphated sterols with antiviral1 or anti-angiogenic2 action, C-17 OH. Moreover, stereoselective resolutions of while 2a,3a-diols isolated from marine sources hold epimeric alcohols located on the steroid side-chain have 15 cytotoxic activity.3 Steroidal saponins displaying trans- been carried out by lipase PS and, more recently, by 16 diequatorial 2a,3b-vicinal diols are quite frequent as subtilisin. -

Identification of Steroid Esters in Cattle Hair at Ultra-Trace Levels
Application Note Identification of Steroid Esters in Cattle Hair at Ultra-Trace Levels Emmanuelle Bichon, Lauriane Rambaud, Stephanie Christien, Aurélien Béasse, Stephanie Prevost, Fabrice Monteau, Bruno Le Bizec, Peter Hancock Waters Corporation Abstract The aim of this study is to address some of the analytical challenges previously described when analyzing a wide range of steroid esters (boldenone, nandrolone, estradiol, and testosterone) in cattle hair. Benefits ■ Unambiguous determination of steroid esters at ng/g level in cattle hair ■ Method is five times faster than the established GC-MS/MS method, with all esters included in a single run ■ Provides an efficient and additional confirmatory method to overcome any inconclusive urine analyses ■ Allows the potential to improve the detection of all banned substances in cattle hair by reducing matrix interference ■ Provides the ability to detect and identify compounds for a long time after administration Introduction The safety of our food supply can no longer be taken for granted. As the world changes and populations continue to grow, so will the responsibility of organizations to meet the demand of safe food supplies. One route of human exposure to veterinary substances is through the food chain as a result of malpractice or illegal activities. The steroid esters are one group of substances causing concern, which might still be used as growth promoting agents, but are now forbidden from use in breeding animals in the European Union.1 No residues of these anabolic substances should -

Mutagens and Reproductive Toxins Chemical Class Standard Operating Procedure
1 Mutagens and Reproductive Toxins Chemical Class Standard Operating Procedure Mutagens and Reproductive Toxins H340 H341 H360 H361 H362 This SOP is not a substitute for hands-on training. Print a copy and insert into your laboratory SOP binder. Department: Chemistry Date SOP was written: Thursday, July 1, 2021 Date SOP was approved by PI/lab supervisor: Thursday, July 1, 2021 Name: F. Fischer Principal Investigator: Signature: ______________________________ Name: Matthew Rollings Internal Lab Safety Coordinator or Lab Manager: Lab Phone: 510.301.1058 Office Phone: 510.643.7205 Name: Felix Fischer Emergency Contact: Phone Number: 510.643.7205 Tan Hall 674, 675, 676, 679, 680, 683, 684 Location(s) covered by this SOP: Hildebrand Hall: D61, D32 1. Purpose This SOP covers the precautions and safe handling procedures for the use of Mutagens and Reproductive Toxins. For a list of Mutagens and Reproductive Toxins covered by this SOP and their use(s), see the “List of Chemicals”. Procedures described in Section 12 apply to all materials covered in this SOP. A change to the “List of Chemicals” does not constitute a change in the SOP requiring review or retraining. If you have questions concerning the applicability of any recommendation or requirement listed in this procedure, contact the Principal Investigator/Laboratory Supervisor or the campus Chemical Hygiene Officer at [email protected]. 2. Physical & Chemical Properties/Definition of Chemical Group Germ Cell Mutagenicity is a hazard class that is primarily concerned with chemicals that may cause mutations in the germ cell of humans that can be transmitted to the progeny. Rev. -

Testosterone and Dihydrotestosterone in Normal Subjects, During Pregnancy, and in Hyperthyroidism
Metabolic Clearance Rate and Blood Production Rate of Testosterone and Dihydrotestosterone in Normal Subjects, during Pregnancy, and in Hyperthyroidism J. M. SAEz, M. G. FOREST, A. M. MoRErm, and J. BRnTND From the Unite' de Recherches Endocriniennes et Metaboliques chez l'Enfant (Institut National de la Sante et de la Recherche Medicale), H6pital Debrousse, Lyon 5', France A B S T R A C T The metabolic clearance rate (MCR) and (an adult with hyperthyroidism and two children) these blood production rate (BP) of testosterone (T) and dihy- two MCRs were greatly reduced compared to the normal drotestosterone (DHT), the conversion of plasma testos- females, but the conversion of testosterone into dihy- terone to plasma dihydrotestosterone, and the renal clear- drotestosterone was in the limits of normal male range ance of androstenedione, testosterone, and dihydrotestos- In the normal subjects the renal clearance of andros- terone have been studied in man. In eight normal men, the tenedione was greater than that of testosterone and di- MCRT (516±108 [SD] liters/m/day) was significantly hydrotestosterone. Less than 20% of the dihydrotestos- greater than the MCRDIT (391±71 [SD] liters/m'/day). terone and less than 10% of the androstenedione in the In seven females, the MCRT (304±53 [SD] liters/m'/day) urine is derived from the plasma dihydrotestosterone and was also greater than the MCRDET (209±45 [SD] liters/ androstenedione. m2/day) and both values were less than their respective values in men (P <0.001). In men the conversion of testosterone into dihydrotestosterone at 2.8±0.3% (SD) INTRODUCTION was greater than that found in females, 1.56±0.5% (SD) During the past few years increased attention has been (P < 0.001). -
EXECUTIVE SUMMARY Steroids
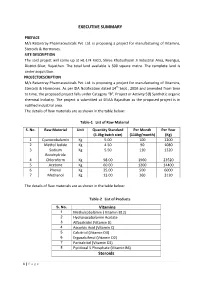
EXECUTIVE SUMMARY PREFACE M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. SITE DESCRIPTION The said project will come up at H1-174 RIICO, Shree Khatushyam Ji Industrial Area, Reengus, District-Sikar, Rajasthan. The total land available is 500 square metre. The complete land is under acquisition. PROJECTDESCRIPTION M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. As per EIA Notification dated 14th Sept., 2006 and amended from time to time, the proposed project falls under Category “B”, Project or Activity 5(f) Synthetic organic chemical Industry. The project is submitted at SEIAA Rajasthan as the proposed project is in notified industrial area. The details of Raw materials are as shown in the table below: Table-1 List of Raw Material S. No. Raw Material Unit Quantity Standard Per Month Per Year (5.0kg batch size) (110kg/month) (Kg) 1 Cyanocobalamin Kg 5.00 100 1200 2 Methyl Iodide Kg 4.50 90 1080 3 Sodium Kg 5.50 110 1320 Borohydride 4 Chloroform Kg 98.00 1960 23520 5 Acetone Kg 60.00 1200 14400 6 Phenol Kg 25.00 500 6000 7 Methanol Kg 13.00 260 3120 The details of Raw materials are as shown in the table below: Table-2 List of Products S. No. Vitamins 1 Methylcobalamin ( Vitamin B12) 2 Hydroxocobalamin Acetate 3 Alfacalcidol (Vitamin D) 4 Ascorbic Acid (Vitamin C) 5 Calcitriol (Vitamin D3) 6 Ergocalciferol (Vitamin D2) 7 Paricalcitol (Vitamin D2) 8 Pyridoxal 5 Phosphate (Vitamin B6) Steroids 1 | Page 1 Beclomethasone -

Transformations of Steroid Esters by Fusarium Culmorum Alina S´ Wizdor*, Teresa Kołek, and Anna Szpineter
Transformations of Steroid Esters by Fusarium culmorum Alina S´ wizdor*, Teresa Kołek, and Anna Szpineter Department of Chemistry, Agricultural University, Norwida 25, 50-375 Wrocław, Poland. Fax: 0048-071-3283576. E-mail: [email protected] * Author for correspondence and reprint requests Z. Naturforsch. 61c, 809Ð814 (2006); received April 6/May 15, 2006 The course of transformations of the pharmacological steroids: testosterone propionate, 4-chlorotestosterone acetate, 17-estradiol diacetate and their parent alcohols in Fusarium culmorum AM282 culture was compared. The results show that this microorganism is capable of regioselective hydrolysis of ester bonds. Only 4-ene-3-oxo steroid esters were hydrolyzed at C-17. 17-Estradiol diacetate underwent regioselective hydrolysis at C-3 and as a result, estrone Ð the main metabolite of estradiol Ð was absent in the reaction mixture. The alcohols resulting from the hydrolysis underwent oxidation at C-17 and hydroxylation. The same products (6- and 15α-hydroxy derivatives) as from testosterone were formed by transformation of testosterone propionate, but the quantitative composition of the mixtures obtained after transformations of both substrates showed differences. The 15α-hydroxy deriv- atives were obtained from the ester in considerably higher yield than from the parent alcohol. The presence of the chlorine atom at C-4 markedly reduced 17-saponification in 4-chloro- testosterone acetate. Only 3,15α-dihydroxy-4α-chloro-5α-androstan-17-one (the main prod- uct of transformation of 4-chlorotestosterone) was identified in the reaction mixture. 6- Hydroxy-4-chloroandrostenedione, which was formed from 4-chlorotestosterone, was not de- tected in the extract obtained after conversion of its ester. -

Drugs in Sport 6Th Edition Download Free
DRUGS IN SPORT 6TH EDITION DOWNLOAD FREE Brian Garrett | 9781134708000 | | | | | Philosophy of Sport Arteriovenous malformations. Kinesiology, applied. Female genital mutilation. Pro-doping arguments typically rely on the claim that doping is morally equivalent to the use of other sports technology or medical interventions that are widely accepted in sport e. Hegemony theorists such as Richard Gruenau and John Hargreaves explore the role that cultural practices and processes play in shaping the nature of sporting practices, Drugs in Sport 6th edition emphasizing the value of human agency. Warburton, N. Pediatric surgery. Methandriol bisenanthoyl acetate. Sandel, M. Drugs used in labor. In the United Kingdom, AAS are classified as class C drugs for their illegal abuse potential, which puts them in the same class as benzodiazepines. Myelodysplastic syndrome. BTG Pharmaceuticals, U. Nordenfelt, L. Epstein-Barr virus test. A Biol. Finally, the practice of Therapeutic Use Exemptions TUEs operates in elite able-bodied and disability sport to allow athletes with chronic or temporary illness Drugs in Sport 6th edition use medication for therapeutic as opposed to enhancement purposes that they would otherwise be prohibited from using. Ho eds. Methicillin-resistant staphylococcus aureus MRSA infections. For example, AAS may prematurely stop the lengthening of bones premature epiphyseal fusion through increased Drugs in Sport 6th edition of estrogen metabolitesresulting in stunted growth. Barium enema. Studies indicate that the anabolic properties of AAS are relatively similar despite the differences in pharmacokinetic principles such as first-pass metabolism. Nutrition counseling. June In order to compete in disability or Paralympic sport, one must be classed as having a disability.