Chapter 7: the Root System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biological Diversity
From the Editors’ Desk….. Biodiversity, which is defined as the variety and variability among living organisms and the ecological complexes in which they occur, is measured at three levels – the gene, the species, and the ecosystem. Forest is a key element of our terrestrial ecological systems. They comprise tree- dominated vegetative associations with an innate complexity, inherent diversity, and serve as a renewable resource base as well as habitat for a myriad of life forms. Forests render numerous goods and services, and maintain life-support systems so essential for life on earth. India in its geographical area includes 1.8% of forest area according to the Forest Survey of India (2000). The forests cover an actual area of 63.73 million ha (19.39%) and consist of 37.74 million ha of dense forests, 25.51 million ha of open forest and 0.487 million ha of mangroves, apart from 5.19 million ha of scrub and comprises 16 major forest groups (MoEF, 2002). India has a rich and varied heritage of biodiversity covering ten biogeographical zones, the trans-Himalayan, the Himalayan, the Indian desert, the semi-arid zone(s), the Western Ghats, the Deccan Peninsula, the Gangetic Plain, North-East India, and the islands and coasts (Rodgers; Panwar and Mathur, 2000). India is rich at all levels of biodiversity and is one of the 12 megadiversity countries in the world. India’s wide range of climatic and topographical features has resulted in a high level of ecosystem diversity encompassing forests, wetlands, grasslands, deserts, coastal and marine ecosystems, each with a unique assemblage of species (MoEF, 2002). -

Transcript Profiling of a Novel Plant Meristem, the Monocot Cambium
Journal of Integrative JIPB Plant Biology Transcript profiling of a novel plant meristem, the monocot cambiumFA Matthew Zinkgraf1,2, Suzanne Gerttula1 and Andrew Groover1,3* 1. US Forest Service, Pacific Southwest Research Station, Davis, California, USA 2. Department of Computer Science, University of California, Davis, USA 3. Department of Plant Biology, University of California, Davis, USA Article *Correspondence: Andrew Groover ([email protected]) doi: 10.1111/jipb.12538 Abstract While monocots lack the ability to produce a xylem tissues of two forest tree species, Populus Research vascular cambium or woody growth, some monocot trichocarpa and Eucalyptus grandis. Monocot cambium lineages evolved a novel lateral meristem, the monocot transcript levels showed that there are extensive overlaps cambium, which supports secondary radial growth of between the regulation of monocot cambia and vascular stems. In contrast to the vascular cambium found in woody cambia. Candidate regulatory genes that vary between the angiosperm and gymnosperm species, the monocot monocot and vascular cambia were also identified, and cambium produces secondary vascular bundles, which included members of the KANADI and CLE families involved have an amphivasal organization of tracheids encircling a in polarity and cell-cell signaling, respectively. We suggest central strand of phloem. Currently there is no information that the monocot cambium may have evolved in part concerning the molecular genetic basis of the develop- through reactivation of genetic mechanisms involved in ment or evolution of the monocot cambium. Here we vascular cambium regulation. report high-quality transcriptomes for monocot cambium Edited by: Chun-Ming Liu, Institute of Crop Science, CAAS, China and early derivative tissues in two monocot genera, Yucca Received Feb. -

Tansley Review Evolution of Development of Vascular Cambia and Secondary Growth
New Phytologist Review Tansley review Evolution of development of vascular cambia and secondary growth Author for correspondence: Rachel Spicer1 and Andrew Groover2 Andrew Groover 1The Rowland Institute at Harvard, Cambridge, MA, USA; 2Institute of Forest Genetics, Pacific Tel: +1 530 759 1738 Email: [email protected] Southwest Research Station, USDA Forest Service, Davis, CA, USA Received: 29 December 2009 Accepted: 14 February 2010 Contents Summary 577 V. Evolution of development approaches for the study 587 of secondary vascular growth I. Introduction 577 VI. Conclusions 589 II. Generalized function of vascular cambia and their 578 developmental and evolutionary origins Acknowledgements 589 III. Variation in secondary vascular growth in angiosperms 581 References 589 IV. Genes and mechanisms regulating secondary vascular 584 growth and their evolutionary origins Summary New Phytologist (2010) 186: 577–592 Secondary growth from vascular cambia results in radial, woody growth of stems. doi: 10.1111/j.1469-8137.2010.03236.x The innovation of secondary vascular development during plant evolution allowed the production of novel plant forms ranging from massive forest trees to flexible, Key words: forest trees, genomics, Populus, woody lianas. We present examples of the extensive phylogenetic variation in sec- wood anatomy, wood formation. ondary vascular growth and discuss current knowledge of genes that regulate the development of vascular cambia and woody tissues. From these foundations, we propose strategies for genomics-based research in the evolution of development, which is a next logical step in the study of secondary growth. I. Introduction this pattern characterizes most extant forest trees, significant variation exists among taxa, ranging from extinct woody Secondary vascular growth provides a means of radially lycopods and horsetails with unifacial cambia (Cichan & thickening and strengthening plant axes initiated during Taylor, 1990; Willis & McElwain, 2002), to angiosperms primary, or apical growth. -

Chapter 5: the Shoot System I: the Stem
Chapter 5 The Shoot System I: The Stem THE FUNCTIONS AND ORGANIZATION OF THE SHOOT SYSTEM PRIMARY GROWTH AND STEM ANATOMY Primary Tissues of Dicot Stems Develop from the Primary Meristems The Distribution of the Primary Vascular Bundles Depends on the Position of Leaves Primary Growth Differs in Monocot and Dicot Stems SECONDARY GROWTH AND THE ANATOMY OF WOOD Secondary Xylem and Phloem Develop from Vascular Cambium Wood Is Composed of Secondary Xylem Gymnosperm Wood Differs from Angiosperm Wood Bark Is Composed of Secondary Phloem and Periderm Buds Are Compressed Branches Waiting to Elongate Some Monocot Stems Have Secondary Growth STEM MODIFICATIONS FOR SPECIAL FUNCTIONS THE ECONOMIC VALUE OF WOODY STEMS SUMMARY ECONOMIC BOTANY: How Do You Make A Barrel? 1 KEY CONCEPTS 1. The shoot system is composed of the stem and its lateral appendages: leaves, buds, and flowers. Leaves are arranged in different patterns (phyllotaxis): alternate, opposite, whorled, and spiral. 2. Stems provide support to the leaves, buds, and flowers. They conduct water and nutrients and produce new cells in meristems (shoot apical meristem, primary and secondary meristems). 3. Dicot stems and monocot stems are usually different. Dicot stems tend to have vascular bundles distributed in a ring, whereas in monocot stems they tend to be scattered. 4. Stems are composed of the following: epidermis, cortex and pith, xylem and phloem, and periderm. 5. Secondary xylem is formed by the division of cells in the vascular cambium and is called wood. The bark is composed of all of the tissues outside the vascular cambium, including the periderm (formed from cork cambium) and the secondary phloem. -
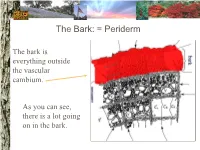
The Bark: = Periderm
The Bark: = Periderm The bark is everything outside the vascular cambium. As you can see, there is a lot going on in the bark. The Bark: periderm: phellogen (cork cambium): The phellogen is the region of cell division that forms the periderm tissues. Phellogen development influences bark appearance. The Bark: periderm: phellem (cork): Phellem replaces the epidermis as the tree increases in girth. Photosynthesis can take place in some trees both through the phellem and in fissures. The Bark: periderm: phelloderm: Phelloderm is active parenchyma tissue. Parenchyma cells can be used for storage, photosynthesis, defense, and even cell division! The Bark: phloem: Phloem tissue makes up the inner bark. However, it is vascular tissue formed from the vascular cambium. The Bark: phloem: sieve tube elements: Sieve tube elements actively transport photosynthates down the stem. Conifers have sieve cells instead. The cambium: The cambium is the primary meristem producing radial growth. It forms the phloem & xylem. The Xylem (wood): The xylem includes everything inside the vascular cambium. The Xylem: a growth increment (ring): The rings seen in many trees represent one growth increment. Growth rings provide the texture seen in wood. The Xylem: vessel elements: Hardwood species have vessel elements in addition to trachieds. Notice their location in the growth rings of this tree The Xylem: fibers: Fibers are cells with heavily lignified walls making them stiff. Many fibers in sapwood are alive at maturity and can be used for storage. The Xylem: axial parenchyma: Axial parenchyma is living tissue! Remember that parenchyma cells can be used for storage and cell division. -

Tree Anatomy Stems and Branches
Tree Anatomy Series WSFNR14-13 Nov. 2014 COMPONENTSCOMPONENTS OFOF PERIDERMPERIDERM by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Around tree roots, stems and branches is a complex tissue. This exterior tissue is the environmental face of a tree open to all sorts of site vulgarities. This most exterior of tissue provides trees with a measure of protection from a dry, oxidative, heat and cold extreme, sunlight drenched, injury ridden site. The exterior of a tree is both an ecological super highway and battle ground – comfort and terror. This exterior is unique in its attributes, development, and regeneration. Generically, this tissue surrounding a tree stem, branch and root is loosely called bark. The tissues of a tree, outside or more exterior to the xylem-containing core, are varied and complexly interwoven in a relatively small space. People tend to see and appreciate the volume and physical structure of tree wood and dismiss the remainder of stem, branch and root. In reality, tree life is focused within these more exterior thin tissue sets. Outside of the cambium are tissues which include transport cells, structural support cells, generation cells, and cells positioned to help, protect, and sustain other cells. All of this life is smeared over the circumference of a predominately dead physical structure. Outer Skin Periderm (jargon and antiquated term = bark) is the most external of tree tissues providing protection, water conservation, insulation, and environmental sensing. Periderm is a protective tissue generated over and beyond live conducting and non-conducting cells of the food transport system (phloem). -

Secondary Thickening Vascular Cambium
nd th Plant Anatomy/ 2 class 12 lecture : Secondary thickening Secondary Thickening Any arising in plant thickness occur far away from the Apies occur as a result of secondary tissues formation & it represent secondary plant body. Secondary thickening occur in most of dicotyledonae & Gymnospermae & some of monocotyledonae as like as in Palmaceae. Secondary thickening occur as a result of 2 kinds of secondary meristematic and they are: 1- Cork cambium (explained in Periderm sub.) 2- Vascular cambium. Vascular cambium: The lateral meristem that forms the secondary vascular tissues, it is located between the xylem & phloem in the stem & root, cylinder in shape, in most petioles & leaf veins it appears as strips. Vascular cambium cells characteristic are: 1- thin cell wall plasmodesmata, dense cytoplasm, dense endoplasmic reticulum, with many rhibosomes. 2- contain (1) nucleus its size in fusiform initials larger than in ray initials. 3- cambium cells appear in radial arrangement with the cell that produce it. 4- usually divide periclinal division and sometimes divide anti linal division. Vascular cambium consist of 2 kind of cells: 1- Fusiform initials / cell: elongated cells with tapering ends (spindle – shaped). 2- Ray cell/ initials: small, isodiametric cells. 1 nd th Plant Anatomy/ 2 class 12 lecture : Secondary thickening Factors that effect on vascular cambium activity: (1)photoperiod, (2)temperature, and (3) water available. The Root The underground part of plant axis specialized as an absorbing & anchoring organ. Origin of the root is the radical which initiate from the hypocotyl of the embryo. The root functions are the absorption of water and other substances anchoring the plant in the substrate, store of vegetative reproduction. -

Dicot/Monocot Root Anatomy the Figure Shown Below Is a Cross Section of the Herbaceous Dicot Root Ranunculus. the Vascular Tissu
Dicot/Monocot Root Anatomy The figure shown below is a cross section of the herbaceous dicot root Ranunculus. The vascular tissue is in the very center of the root. The ground tissue surrounding the vascular cylinder is the cortex. An epidermis surrounds the entire root. The central region of vascular tissue is termed the vascular cylinder. Note that the innermost layer of the cortex is stained red. This layer is the endodermis. The endodermis was derived from the ground meristem and is properly part of the cortex. All the tissues inside the endodermis were derived from procambium. Xylem fills the very middle of the vascular cylinder and its boundary is marked by ridges and valleys. The valleys are filled with phloem, and there are as many strands of phloem as there are ridges of the xylem. Note that each phloem strand has one enormous sieve tube member. Outside of this cylinder of xylem and phloem, located immediately below the endodermis, is a region of cells called the pericycle. These cells give rise to lateral roots and are also important in secondary growth. Label the tissue layers in the following figure of the cross section of a mature Ranunculus root below. 1 The figure shown below is that of the monocot Zea mays (corn). Note the differences between this and the dicot root shown above. 2 Note the sclerenchymized endodermis and epidermis. In some monocot roots the hypodermis (exodermis) is also heavily sclerenchymized. There are numerous xylem points rather than the 3-5 (occasionally up to 7) generally found in the dicot root. -

4Th Convention: SFE – INDIA, 2017
PROGRAMME 4th Convention: SFE – INDIA, 2017 National Symposium “Ashwagandha” & Ethnopharmacology Conclave on Uses of Medicinal Plants by Traditional Healers of India – Local Heath Tradition September 09-10, 2017 Organized by: School of Natural Product Studies Jadavpur University, Kolkata, India web: www.jaduniv.edu.in In Association with: Society for Ethnopharmacology (SFE - INDIA) 23/3 Saktigarh, Kolkata www.ethnopharmacology.in Venue: Jadavpur University, Kolkata 4th National Convention of Society for Ethnopharmacology, India (SFE - INDIA) is being organized by the School of Natural Product Studies (SNPS), Jadavpur University during September 09-10, 2017. The theme of the convention is focused on “Ashwagandha” and Uses of Medicinal Plants by the Traditional healers of India – Local Heath Tradition”. On behalf of the School of Natural Product Studies and the organizing committee, I would like to convey my warm welcome to you all for the 4th convention of SFE -INDIA. With the history of one of the oldest civilization harbors many traditional alternative and complementary medicines for the health care, India has a rich heritage on use of Traditional medicine in healthcare. Botanicals serve as the source of therapeutically active molecules for many years. Ashwagandha (Withania somnifera), one of the most popular Indian medicinal plants and also considered to be nature's gift to mankind, has been an important herb in the Ayurvedic and indigenous medical system for over 3000 years. In Ayurveda, Ashwagandha is considered as a “rasayana” herb, which works on a nonspecific basis to increase health and longevity. Ashwagandha has been used to treat variety of diseases and human ailments. This is also a crucial herb that contributes a huge market potential throughout the globe. -

Eudicots Monocots Stems Embryos Roots Leaf Venation Pollen Flowers
Monocots Eudicots Embryos One cotyledon Two cotyledons Leaf venation Veins Veins usually parallel usually netlike Stems Vascular tissue Vascular tissue scattered usually arranged in ring Roots Root system usually Taproot (main root) fibrous (no main root) usually present Pollen Pollen grain with Pollen grain with one opening three openings Flowers Floral organs usually Floral organs usually in in multiples of three multiples of four or five © 2014 Pearson Education, Inc. 1 Reproductive shoot (flower) Apical bud Node Internode Apical bud Shoot Vegetative shoot system Blade Leaf Petiole Axillary bud Stem Taproot Lateral Root (branch) system roots © 2014 Pearson Education, Inc. 2 © 2014 Pearson Education, Inc. 3 Storage roots Pneumatophores “Strangling” aerial roots © 2014 Pearson Education, Inc. 4 Stolon Rhizome Root Rhizomes Stolons Tubers © 2014 Pearson Education, Inc. 5 Spines Tendrils Storage leaves Stem Reproductive leaves Storage leaves © 2014 Pearson Education, Inc. 6 Dermal tissue Ground tissue Vascular tissue © 2014 Pearson Education, Inc. 7 Parenchyma cells with chloroplasts (in Elodea leaf) 60 µm (LM) © 2014 Pearson Education, Inc. 8 Collenchyma cells (in Helianthus stem) (LM) 5 µm © 2014 Pearson Education, Inc. 9 5 µm Sclereid cells (in pear) (LM) 25 µm Cell wall Fiber cells (cross section from ash tree) (LM) © 2014 Pearson Education, Inc. 10 Vessel Tracheids 100 µm Pits Tracheids and vessels (colorized SEM) Perforation plate Vessel element Vessel elements, with perforated end walls Tracheids © 2014 Pearson Education, Inc. 11 Sieve-tube elements: 3 µm longitudinal view (LM) Sieve plate Sieve-tube element (left) and companion cell: Companion cross section (TEM) cells Sieve-tube elements Plasmodesma Sieve plate 30 µm Nucleus of companion cell 15 µm Sieve-tube elements: longitudinal view Sieve plate with pores (LM) © 2014 Pearson Education, Inc. -
+ Complex Tissues
+ Complex Tissues ! Complex tissues are made up of two or more cell types. ! Xylem - Chief conducting tissue for water and minerals absorbed by the roots. ! Vessels - Made of vessel elements. ! Long tubes open at each end. ! Tracheids - Tapered at the ends with pits that allow water passage between cells. ! Rays - Lateral conduction. + Complex Tissues - Xylem ! Tracheids ! Long, thin cells with pointed ends that conduct water vertically ! Line up in columns like pipes by overlapping their tapered ends ! die when reach maturity ! Water conducted through tubes made up of tracheid cell walls ! Wherever two ends join, small holes in the cell wall called pits line up to allow water to flow from one tracheid to another. + Complex Tissues - Xylem ! Pits always occur in pairs so that a pair of pits lines up on either side of the middle lamella, or center layer, of the cell wall. + Complex Tissues - Xylem !Vessel elements !barrel-shaped cells with open ends that conduct water vertically. !line up end to end forming columns, called vessels, that conduct water. !Some have completely open ends, while others have narrow strips of cell wall material that partially covers the ends !Die at maturity, like tracheids. + Complex Tissues - Xylem ! Ray cells………. ! Long lived parenchyma cells that extend laterally like the spokes of a wheel from the center of a woody stem out towards the exterior of the stem ! alive at maturity ! Transport materials horizontally from center outward + Complex Tissues - Xylem ! Xylem fibers – ! long, thin sclerenchyma cells ! Xylem parenchyma cells- that run parallel to the ! Living cells vessel element ! Distributed among tracheids and vessels ! Help strengthen and support xylem ! Store water and nutrients + Complex Tissues - Phloem ! Phloem brings sugar [glucose from photosynthesis] from the leaves to all parts of the plant body. -

Tree Anatomy Stems and Branches
Tree Anatomy: STEM COMPONENTS Dr. Kim D. Coder, Professor of Tree Biology & Health Care, Warnell School, UGA Trees attempt to occupy more space and control available resources through cell divisions and cell expansion. From the end of a shoot tip to the end of a root tip, trees elongate. Along this axis of growth, trees also expand radially, which is termed “secondary growth.” Tree growth is initiated in the shoot tips (growing points / buds), root tips, vascular cambium (i.e. or simply cambium), and phellogen which generates periderm. The first two meristems are primary generation systems and the second two are termed secondary meristems. Cambial activity and xylem formation, as seen as visible increases in growth increment volume are radial growth, and reviewed here. Cross-Section Tree stem anatomy is usually visualized in cross-section. In this form, two-demensional “layers” or “rings,” and tissue types can be identified. More difficult is to visualize these two-dimensional cross- sections and incorporate them into a three dimensional object which changes and grows over time. Moving from outside to inside a stem, tissues encountered include those associated with periderm, secondary cortex, phloem, xylem, and pith. A traditional means of describing a mature tree stem cross-section defines which tissue type is cut first, second and third using a handsaw. A list of generic tissues in stems from outside to inside could include: Figure 1. epidermis and primary cortex = exterior primary protective tissues quickly rent, torn, and discarded with secondary growth (expansion of girth by lateral meristems – vascular cambium and phellogen). periderm = a multiple layer tissue responsible for tree protection and water conservation generated over the exterior of a tree from shoot to root, and produced by the phellogen (cork cambium – lateral meristem).