Guideline on Peripheral Arterial Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification System of the Tibiofibular Syndesmosis Blood Supply and Its
www.nature.com/scientificreports OPEN Classifcation system of the tibiofbular syndesmosis blood supply and its clinical relevance Received: 16 February 2018 Izabela Mróz1, Piotr J. Bachul1,2, Krzysztof A. Tomaszewski1, Tomasz Bereza1, Krzysztof Gil3, Accepted: 7 June 2018 Jerzy A. Walocha1 & Artur Pasternak 1 Published: xx xx xxxx Due to the lack of anatomical studies concerning complexity of the tibiofbular syndesmosis blood supply, density of blood vessels with further organization of syndesmotic vascular variations is presented in clinically relevant classifcation system. The material for the study was obtained from cadaveric dissections. We dissected 50 human ankles observing diferent types of arterial blood supply. Our classifcation system is based on the vascular variations of the anterior aspect of tibiofbular syndesmosis and corresponds with vascular density. According to our study the mean vascular density of tibiofbular syndesmosis is relatively low (4.4%) and depends on the type of blood supply. The highest density was observed among ankles with complete vasculature and the lowest when lateral anterior malleolar artery was absent (5.8% vs. 3.5%, respectively). Awareness of various types of tibiofbular syndesmosis arterial blood supply is essential for orthopedic surgeons who operate in the ankle region and radiologists for the anatomic evaluation of this area. Knowledge about possible variations along with relatively low density of vessels may contribute to modifcation of treatment approach by the increase of the recommended time of syndesmotic screw stabilization in order to prevent healing complications. Tibiofbular syndesmosis is a fbrous connection localized between the fbular notch of the tibia and medial surface of the lateral ankle. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Vascular Anatomy of the Free Fibula Flap Including the Lateral Head of the Soleus Muscle Applied to Maxillo-Mandibular Reconstruction
Surgical and Radiologic Anatomy (2019) 41:447–454 https://doi.org/10.1007/s00276-018-2166-2 ORIGINAL ARTICLE Vascular anatomy of the free fibula flap including the lateral head of the soleus muscle applied to maxillo-mandibular reconstruction Lara Nokovitch1,2 · Julien Davrou2 · François Bidault4 · Bernard Devauchelle2 · Stéphanie Dakpé2 · Christian Vacher3,5 Received: 1 May 2018 / Accepted: 8 December 2018 / Published online: 14 December 2018 © Springer-Verlag France SAS, part of Springer Nature 2018 Abstract Purpose Initially described by Baudet in 1982, the fibula flap including the lateral head of the soleus muscle allows a one- stage reconstruction for large maxillo-mandibular defects. The aim of this study was to evaluate the number of muscular branches destined to the soleus muscle and their distance from the origin of the fibular artery, to assess the vascular anatomy of the free fibula flap including the lateral head of the soleus muscle applied to maxillo-mandibular reconstruction. Methods We performed a cadaveric anatomic study on ten lower limbs, and a CT angiography anatomic study on 38 legs. The number of soleus branches originating from the fibular artery, and the distance between the origin of the fibular artery and each of the identified branches were measured. Results The number of soleus branches destined to the lateral head of the soleus muscle is variable, with in our study 1–3 branches found. Soleus branches destined to the lateral head of the soleus muscle emerged at a distance ranging between 0 and 2.9 cm (mean value = 1.82 cm) from the origin of the fibular artery in 40% of cases, between 3 and 5.9 cm (mean value = 4.27 cm) from the origin of the fibular artery in 37% of cases, and was at a distance of 6 cm or more (mean value = 6.93 cm) from the origin of the fibular artery in 20% of cases. -

Vela Proximal Endograft System Phoenix Atherectomy System
A PREVIEW OF today’s NEW PRODUCTS ONS I Vela Proximal Endograft System Endologix (Irvine, CA) has Endologix announced the United States (949) 595-7200 launch of the FDA-approved INNOVAT www.endologix.com/Vela Vela proximal endograft sys- KEY FEATURES tem, which is designed for • Circumferential graft line marker the treatment of proximal for enhanced visibility aortic neck anatomies during • New delivery system endovascular aneurysm repair. • ActiveSeal technology The Vela system, which was developed with feedback from leading physicians, features a new delivery system and a circumferen- tial graft line marker for enhanced visibility during the implantation procedure. One of the first Vela procedures in the United States was performed by Julio Rodriguez, MD, FACS, a vascular surgeon at the Arizona Heart Institute in Phoenix, Arizona. In the company’s press release, Dr. Rodriguez commented, “The Vela delivery system is very intuitive, and the endograft has excellent visibility.” Phoenix Atherectomy System AtheroMed, Inc. (Menlo Park, CA) AtheroMed, Inc. has received CE Mark approval and FDA (650) 473-6846 510(k) clearance to market the Phoenix www.atheromedinc.com Atherectomy System, a pushable, over-the- KEY FEATURES wire system that uses a rotating, front-cut- • Cut, capture, and clear mechanism ting element located at the distal tip of the of action catheter to shave diseased material directly • Able to treat soft plaque or calcium • Profile down to 5 F into the catheter. The debulked material is • No capital equipment required then continuously captured and removed • Front-cutting, single insertion by an internal Archimedes screw that runs the length of the catheter. -
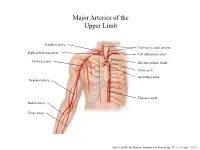
Major Arteries of the Upper Limb
Major Arteries of the Upper Limb Vertebral artery Common carotid arteries Right subclavian artery Left subclavian artery Axillary artery Brachiocephalic trunk Aortic arch Ascending aorta Brachial artery Thoracic aorta Radial artery Ulnar artery Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.23 Major Arteries of the Abdominal Region Renal artery Celiac trunk Abdominal aorta Superior mesenteric artery Gonadal artery Inferior mesenteric artery Common iliac artery Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.24 Common iliac artery Internal iliac artery Major Arteries of the External iliac artery Lower Limb Femoral artery Popliteal artery Anterior tibial artery Fibular artery Posterior tibial artery Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.25 Major Veins of the Upper Limb Internal jugular vein (left) Subclavian vein (right) External jugular vein (left) Axillary vein Brachiocephalic veins Cephalic vein Superior vena cava Brachial vein Basilic vein Median cubital vein Inferior vena cava Radial vein Ulnar vein Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.28 Major Veins of the Abdominal Cavity – Part 1 Hepatic veins Inferior vena cava Renal vein (left) Gonadal vein (left) Gonadal vein (right) Common iliac vein (left) Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.29 Major Veins of the Abdominal Cavity – Part 2 (Hepatic portal circulation) Hepatic portal vein Splenic vein Inferior mesenteric vein Superior mesenteric vein Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.29 Common iliac vein (left) Internal iliac vein Major Veins of the External iliac vein Lower Limb Great saphenous vein Femoral vein Popliteal vein Fibular vein Small saphenous vein Anterior tibial Posterior tibial vein vein Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 19.30 . -

Clinical Anatomy of the Lower Extremity
Государственное бюджетное образовательное учреждение высшего профессионального образования «Иркутский государственный медицинский университет» Министерства здравоохранения Российской Федерации Department of Operative Surgery and Topographic Anatomy Clinical anatomy of the lower extremity Teaching aid Иркутск ИГМУ 2016 УДК [617.58 + 611.728](075.8) ББК 54.578.4я73. К 49 Recommended by faculty methodological council of medical department of SBEI HE ISMU The Ministry of Health of The Russian Federation as a training manual for independent work of foreign students from medical faculty, faculty of pediatrics, faculty of dentistry, protocol № 01.02.2016. Authors: G.I. Songolov - associate professor, Head of Department of Operative Surgery and Topographic Anatomy, PhD, MD SBEI HE ISMU The Ministry of Health of The Russian Federation. O. P.Galeeva - associate professor of Department of Operative Surgery and Topographic Anatomy, MD, PhD SBEI HE ISMU The Ministry of Health of The Russian Federation. A.A. Yudin - assistant of department of Operative Surgery and Topographic Anatomy SBEI HE ISMU The Ministry of Health of The Russian Federation. S. N. Redkov – assistant of department of Operative Surgery and Topographic Anatomy SBEI HE ISMU THE Ministry of Health of The Russian Federation. Reviewers: E.V. Gvildis - head of department of foreign languages with the course of the Latin and Russian as foreign languages of SBEI HE ISMU The Ministry of Health of The Russian Federation, PhD, L.V. Sorokina - associate Professor of Department of Anesthesiology and Reanimation at ISMU, PhD, MD Songolov G.I K49 Clinical anatomy of lower extremity: teaching aid / Songolov G.I, Galeeva O.P, Redkov S.N, Yudin, A.A.; State budget educational institution of higher education of the Ministry of Health and Social Development of the Russian Federation; "Irkutsk State Medical University" of the Ministry of Health and Social Development of the Russian Federation Irkutsk ISMU, 2016, 45 p. -

Beyond Common Femoral Artery Access Exploring a Combined Approach for Treatment of Critical Limb Ischemia
BTK DISEASE Hybrid Procedures for CLI: Beyond Common Femoral Artery Access Exploring a combined approach for treatment of critical limb ischemia. By Jos C. van den Berg, MD, PhD atients with end-stage peripheral artery disease In cases of multilevel disease, the decision on how to typically demonstrate multilevel disease that can combine the three major treatment arms (endovas- extend from the aorta to the infragenicular arter- cular treatment, bypass surgery, and endarterectomy) ies. An evaluation of the lesion location (iliac, fem- should be made based on target lesion complexity Poropopliteal, or infrapopliteal segment) in patients who as well as the underlying condition.5 A hybrid proce- presented with critical limb ischemia (CLI) demonstrated dure can reduce the perioperative risk, especially in that the large majority has involvement of at least two older patients. Distal revascularization in patients with arterial segments.1 The lesion distribution combined with Rutherford category 5 and TASC D lesions, as well as in the concomitant comorbidities have implications for patients with major tissue loss will lead to better limb subsequent treatment, and oftentimes, a pure endovas- salvage, reintervention, and survival rates.6 The major- cular or open surgical procedure is not feasible. To treat ity of reports on the use of hybrid procedures involves the patient with complex multilevel disease in a single simultaneous treatment with endovascular treatment intervention that can provide an extensive revasculariza- of the aortoiliac segment -

Diagnosing and Treating Vascular Disease
THE CENTER FOR VASCULAR CARE AT WASHINGTON HOSPITAL CENTER Diagnosing and Treating Vascular Disease If you have symptoms of vascular disease or Peripheral Artery Disease (PAD), the best thing to do is see your physician for examination and possible testing. And, if you have the following risk factors, see your doctor to determine if you should be tested. The Center for Vascular Care at Washington Hospital Center can provide painless, risk free tests and the most complete array of treatment options for vascular disease in the region. RISK FACTORS TESTING FOR VASCULAR DISEASE FOR VASCULAR DISEASE Ankle-brachial Index: Patients with leg I Cigarette smoking artery blockage are at increased risk of I Older than age 50* heart attack and stroke. This test (ABI) is I Obesity a simple, safe, painless test for blockages. A blood pressure cuff is placed above I Diabetes the ankles and pressures are measured I Heart disease compared to arm pressures to determine I High Cholesterol the risk of cardiovascular events. It takes I High-stress lifestyle 5–10 minutes. I High blood pressure Carotid Duplex Scan: Blockage in a major I Family history of aneurysms artery increases the likelihood of a stroke. *Over age 65 if no other risk factors exist Thickening of the walls of the carotid arteries can lead to heart attack. An arterial SYMPTOMS OF VASCULAR DISEASE duplex scan is a safe, painless, highly I Leg pain that goes away with rest accu rate method of determining blockage. A sound wave device, placed lightly on the I Numbness of the legs or feet at rest skin, can tell if there is a problem. -

Lower Extremity Invasive Diagnostic and Endovascular Procedures
UnitedHealthcare® Commercial Medical Policy Lower Extremity Invasive Diagnostic and Endovascular Procedures Policy Number: 2021T0602F Effective Date: July 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policies Coverage Rationale ........................................................................... 1 • Pneumatic Compression Devices Documentation Requirements......................................................... 2 • Surgical and Ablative Procedures for Venous Definitions ........................................................................................... 3 Insufficiency and Varicose Veins Applicable Codes .............................................................................. 4 Description of Services ..................................................................... 5 Community Plan Policy Clinical Evidence ............................................................................... 5 • Lower Extremity Invasive Diagnostic and U.S. Food and Drug Administration ................................................ 7 Endovascular Procedures References ......................................................................................... 8 Medicare Advantage Coverage Summary Policy History/Revision Information................................................ 9 • Instructions for Use ........................................................................... 9 Cardiovascular Diagnostic Procedures Coverage Rationale Note: This policy does not apply to upper extremities. Diagnosis Lower extremity -

Arteries of the Lower Limb
BLOOD SUPPLY OF LOWER LIMB Ali Fırat Esmer, MD Ankara University Faculty of Medicine Department of Anatomy Abdominal aorta Aortic bifurcation Right common iliac artery Left common iliac artery Right external Left external iliac artery iliac artery Rigt and left internal iliac arteries GLUTEAL REGION Structures passing through the suprapriform foramen Superior gluteal artery and vein Superior gluteal nerve Structures passing through the infrapriform foramen Inferior gluteal artery and vein Inferior gluteal nerve Sciatic nerve Posterior femoral cutaneous nerve Internal pudendal artery and vein Pudendal nerve • Femoral artery is the principal artery of the lower limb • Femoral artery is the continuation of the external iliac artery • External iliac artery becomes the femoral artery as it passes posterior to the inguinal ligament • Femoral artery, first enters the femoral triangle. Leaving the tirangle it passes through the adductor canal and then adductor hiatus and reaches to the popliteal fossa, where it becomes the popliteal artery Contents of the femoral triangle (from lateral to medial) • Femoral nerve (and its branches) • Saphenous nerve (sensory branch of the femoral nerve) • Femoral artery (and its several branches) • Deep femoral artery (deep artery of the thigh) and its branches in this region; medial and lateral circumflex femoral arteries and perforating branches • Femoral vein (and veins draining to its proximal part such as the great saphenous vein and deep femoral vein) • Deep inguinal lymph nodes MUSCULAR AND VASCULAR COMPARTMENTS -

Endovascular Versus Open Revascularization for Peripheral Arterial Disease
COVER STORY Endovascular Versus Open Revascularization for Peripheral Arterial Disease A review of 12-year data revealing changes in amputation and limb salvage rates associated with a shift in revascularization modalities. BY NILESH N. BALAR, MD; RANJITH DODLA, MD; PARIND OZA, MD; PARTH N. PATEL; AND MAYANK PATEL, MD eripheral arterial disease (PAD) affects approxi- mately 12% to 14% of the general population, which steadily increases with age and affects up P to 20% of patients who are older than 75 years.1 The prevalence of PAD markedly increases in patients with diabetes, hypertension, hyperlipidemia, and a history of smoking.2 The most sensitive tool to detect PAD is the ankle-brachial index.2 Various treatment options include lifestyle modifications, endovascular revascularization, and open revascularization. In the past, most of these patients with significant limb ischemia have been treated Figure 1. Trends in endovascular revascularization, surgical with surgical revascularization. However, with rapid revascularization, and amputation over 12 years. advances in catheter-based technology, there has been a significant shift toward endovascular interventions.3,4 lower extremity amputations. All amputations were per- There are very few data regarding limb salvage rates formed by vascular surgeons only. Surgical revasculariza- and lower extremity amputation rates after infrainguinal tion procedures were femoropopliteal artery bypass, endovascular procedures.5 To examine the impact of femorofemoral crossover bypass, femorotibial vessel endovascular interventions on the amputation and limb bypass, and other distal vessel bypasses. Both native and salvage rates and determine its relationship to open prosthetic conduits were included in the study. Because revascularization, we set forth to retrospectively examine few axillary femoral and aortobifemoral bypass proce- a 12-year period of data from this patient population at dures were performed in any given year, these procedures our center. -

Recommended Personal Protective Equipment for Outpatient Management of Asymptomatic Patients
Recommended Personal Protective Equipment for Outpatient Management of Asymptomatic Patients These recommendations represent the required PPE for a variety of visits with differential risk. Providers should follow Standard and Transmission-Based Precautions in addition to the precautions recommended in the Ambulatory Infection Prevention Policy. Pre- Risk- PPE Recommendation Physical Room Closure Procedure Description/Examples stratification Test Environment Requirement Patient* Provider Exams during which the patient can remain masked for the majority of the visit and there is not a prolonged oropharyngeal or nasopharyngeal exam performed such as: • New patient or follow-up patient visits in primary care • New patient or follow up patient visits in specialty care not included in med/high risk categories • Exercise Stress Test** • Neurodiagnostic Testing Promote physical Low No • Gynecologic procedures Face mask Face mask distancing in exam None • Urologic procedures rooms during history • Injections: Joint, Spine, Pain • Endovascular procedures (angioplasty, aortogram, HD access, atherectomy, sclerotherapy, endovascular laser ablation • Cardiac Catheterization lab procedures • Pulmonary rehab** • PT/OT** Exams during which the patient will be unmasked for a prolonged period of time, the patient is unable to wear a mask or during which the provider is required to be in close proximity to a masked patient’s face for a prolonged period of time including: • Prolonged oropharyngeal or nasopharyngeal exam Face mask Promote physical Face mask