Vela Proximal Endograft System Phoenix Atherectomy System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diagnosing and Treating Vascular Disease
THE CENTER FOR VASCULAR CARE AT WASHINGTON HOSPITAL CENTER Diagnosing and Treating Vascular Disease If you have symptoms of vascular disease or Peripheral Artery Disease (PAD), the best thing to do is see your physician for examination and possible testing. And, if you have the following risk factors, see your doctor to determine if you should be tested. The Center for Vascular Care at Washington Hospital Center can provide painless, risk free tests and the most complete array of treatment options for vascular disease in the region. RISK FACTORS TESTING FOR VASCULAR DISEASE FOR VASCULAR DISEASE Ankle-brachial Index: Patients with leg I Cigarette smoking artery blockage are at increased risk of I Older than age 50* heart attack and stroke. This test (ABI) is I Obesity a simple, safe, painless test for blockages. A blood pressure cuff is placed above I Diabetes the ankles and pressures are measured I Heart disease compared to arm pressures to determine I High Cholesterol the risk of cardiovascular events. It takes I High-stress lifestyle 5–10 minutes. I High blood pressure Carotid Duplex Scan: Blockage in a major I Family history of aneurysms artery increases the likelihood of a stroke. *Over age 65 if no other risk factors exist Thickening of the walls of the carotid arteries can lead to heart attack. An arterial SYMPTOMS OF VASCULAR DISEASE duplex scan is a safe, painless, highly I Leg pain that goes away with rest accu rate method of determining blockage. A sound wave device, placed lightly on the I Numbness of the legs or feet at rest skin, can tell if there is a problem. -

Lower Extremity Invasive Diagnostic and Endovascular Procedures
UnitedHealthcare® Commercial Medical Policy Lower Extremity Invasive Diagnostic and Endovascular Procedures Policy Number: 2021T0602F Effective Date: July 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policies Coverage Rationale ........................................................................... 1 • Pneumatic Compression Devices Documentation Requirements......................................................... 2 • Surgical and Ablative Procedures for Venous Definitions ........................................................................................... 3 Insufficiency and Varicose Veins Applicable Codes .............................................................................. 4 Description of Services ..................................................................... 5 Community Plan Policy Clinical Evidence ............................................................................... 5 • Lower Extremity Invasive Diagnostic and U.S. Food and Drug Administration ................................................ 7 Endovascular Procedures References ......................................................................................... 8 Medicare Advantage Coverage Summary Policy History/Revision Information................................................ 9 • Instructions for Use ........................................................................... 9 Cardiovascular Diagnostic Procedures Coverage Rationale Note: This policy does not apply to upper extremities. Diagnosis Lower extremity -

Endovascular Versus Open Revascularization for Peripheral Arterial Disease
COVER STORY Endovascular Versus Open Revascularization for Peripheral Arterial Disease A review of 12-year data revealing changes in amputation and limb salvage rates associated with a shift in revascularization modalities. BY NILESH N. BALAR, MD; RANJITH DODLA, MD; PARIND OZA, MD; PARTH N. PATEL; AND MAYANK PATEL, MD eripheral arterial disease (PAD) affects approxi- mately 12% to 14% of the general population, which steadily increases with age and affects up P to 20% of patients who are older than 75 years.1 The prevalence of PAD markedly increases in patients with diabetes, hypertension, hyperlipidemia, and a history of smoking.2 The most sensitive tool to detect PAD is the ankle-brachial index.2 Various treatment options include lifestyle modifications, endovascular revascularization, and open revascularization. In the past, most of these patients with significant limb ischemia have been treated Figure 1. Trends in endovascular revascularization, surgical with surgical revascularization. However, with rapid revascularization, and amputation over 12 years. advances in catheter-based technology, there has been a significant shift toward endovascular interventions.3,4 lower extremity amputations. All amputations were per- There are very few data regarding limb salvage rates formed by vascular surgeons only. Surgical revasculariza- and lower extremity amputation rates after infrainguinal tion procedures were femoropopliteal artery bypass, endovascular procedures.5 To examine the impact of femorofemoral crossover bypass, femorotibial vessel endovascular interventions on the amputation and limb bypass, and other distal vessel bypasses. Both native and salvage rates and determine its relationship to open prosthetic conduits were included in the study. Because revascularization, we set forth to retrospectively examine few axillary femoral and aortobifemoral bypass proce- a 12-year period of data from this patient population at dures were performed in any given year, these procedures our center. -

Recommended Personal Protective Equipment for Outpatient Management of Asymptomatic Patients
Recommended Personal Protective Equipment for Outpatient Management of Asymptomatic Patients These recommendations represent the required PPE for a variety of visits with differential risk. Providers should follow Standard and Transmission-Based Precautions in addition to the precautions recommended in the Ambulatory Infection Prevention Policy. Pre- Risk- PPE Recommendation Physical Room Closure Procedure Description/Examples stratification Test Environment Requirement Patient* Provider Exams during which the patient can remain masked for the majority of the visit and there is not a prolonged oropharyngeal or nasopharyngeal exam performed such as: • New patient or follow-up patient visits in primary care • New patient or follow up patient visits in specialty care not included in med/high risk categories • Exercise Stress Test** • Neurodiagnostic Testing Promote physical Low No • Gynecologic procedures Face mask Face mask distancing in exam None • Urologic procedures rooms during history • Injections: Joint, Spine, Pain • Endovascular procedures (angioplasty, aortogram, HD access, atherectomy, sclerotherapy, endovascular laser ablation • Cardiac Catheterization lab procedures • Pulmonary rehab** • PT/OT** Exams during which the patient will be unmasked for a prolonged period of time, the patient is unable to wear a mask or during which the provider is required to be in close proximity to a masked patient’s face for a prolonged period of time including: • Prolonged oropharyngeal or nasopharyngeal exam Face mask Promote physical Face mask -

Incidence of Procedural Myocardial Infarction and Cardiac Magnetic
SHORT REPORT CORONARY INTERVENTIONS Euro Intervention 2018;14: 819-823 Incidence of procedural myocardial infarction and cardiac online published magnetic resonance imaging-detected myocardial injury following percutaneous coronary intervention with rotational atherectomy May 2018 Margaret B. McEntegart1,2*, MD, PhD; David Corcoran1, MD; David Carrick1, MD; Guillaume Clerfond2, MD; Novalia Sidik2, MD; Damien Collison1, MD; Keith R. Robertson1, MD; Aadil Shaukat1, MD; Stuart Watkins1, MD; Paul R. Rocchicholi1, MD; Hany Eteiba1, MD; Mark P. Petrie1,2, MD; Mitchell M. Lindsay1, MD; Keith G. Oldroyd1,2, MD; Colin Berry1,2, MD, PhD 1. Golden Jubilee National Hospital, Clydebank, United Kingdom; 2. BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom Introduction Methods Rotational atherectomy (RA) is the most commonly used tech- This was a prospective cohort study conducted at a regional heart nique to modify calcific coronary artery disease (CAD) to facil- centre in the United Kingdom. We recruited 58 near-consecutive, itate stent implantation and deployment. While embolisation of eligible patients with stable angina requiring PCI with RA to a cal- fragmented plaque into the microcirculation during RA is thought cific coronary lesion. to be associated with a higher incidence of procedural myocar- The exclusion criteria were: MI within prior seven days; Q-waves dial infarction (type 4a MI), this has never been comprehensively on ECG, wall motion abnormality (WMA) on echocardiogram, or demonstrated1,2. infarct on baseline CMR in target vessel territory; contraindica- The definition of type 4a MI is contentious with two defini- tion to CMR; chronic total occlusion (CTO), and eGFR <30 ml/ tions, the third universal definition (3rd UD) and the Society for min/1.73 m2. -

Coronary Atherectomy: a Current Assessment
CURRENT TRENDS IN PCI Coronary Atherectomy: A Current Assessment A contemporary review of atherectomy devices for treating calcified coronary artery lesions. BY EVAN SHLOFMITZ, DO n one of the initial articles on interventional cardiology, compliance may allow for complete stent expansion and Gruntzig et al noted, “At present, the technic [sic] is lead to improved procedural success. Mild to moderately limited by anatomic factors, such as vessel tortuosity… calcified lesions can often be managed with noncom- and calcified stenosis.”1 Despite the many advances in pliant balloons with high-pressure inflations, as well as Ithe field, these words proved to be prophetic, as coronary with cutting, scoring, and sculpting balloons. However, artery calcification continues to pose many challenges to moderate to severely calcified lesions often require an successful percutaneous coronary intervention (PCI). atherectomy strategy for optimal lesion preparation. Coronary artery calcification increases the complexity of Following atherectomy, stent delivery should utilize the PCI, with less favorable results than in noncalcified lesions. latest-generation drug-eluting stent, whenever possible, Severely calcified lesions increase the risk of dissection, to minimize restenosis. inhibit stent delivery and adequate stent expansion, and The atherectomy devices that are currently commercially are prone to stent malapposition with insufficient drug available differ by design and mechanism of action. The penetration.2-6 These factors may contribute to increased -
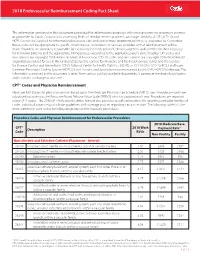
2018 Endovascular Reimbursement Coding Fact Sheet
2018 Endovascular Reimbursement Coding Fact Sheet The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment, or charge. Similarly, all CPT, ICD-10 and HCPCS codes are supplied for informational purposes only and represent no statement, promise, or guarantee by Cordis that these codes will be appropriate to specific circumstances or products or services provided or that reimbursement will be made. Providers are ultimately responsible for exercising their independent clinical judgment to determine medical necessity for individual patients and the appropriate billing process according to the applicable payer’s current policy. CPT codes and descriptions are copyright 2018 American Medical Association. ICD-10 codes and descriptions are copyright 2018 World Health Organization; revised for use in the United States by the Centers for Medicare and Medicaid Services (CMS) and the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics (NCHS) as ICD-10-CM / ICD-10-PCS. Healthcare Common Procedure Coding System (HCPCS) Level II codes and descriptions are maintained by the CMS HCPCS Workgroup. The information contained in this document is taken from various publicly available documents, is current at the date of publication and is subject to change at any time. CPT® Codes and Physician Reimbursement Medicare Part B pays for physician services based upon the Medicare Physician Fee Schedule (MPFS). Fee schedule amounts are calculated according to the Resource-Based Relative Value Scale (RBRVS), which is updated each year. Procedures are reported using CPT® codes.1 The 2018 CPT Professional Edition Manual also provides specific instructions for reporting particular families of codes. -

Complete Guide for Interventional Radiology Page an In-Depth Guide to Interventional Radiology Coding, Billing and Reimbursement for Facilities and Physicians
2019 Complete Guide for Interventional Radiology page An in-depth guide to interventional radiology coding, billing and reimbursement for facilities and physicians Sample Power up your coding optum360coding.com FIR_FIR19_CVR.indd 1 11/27/17 2:14 PM Contents Introduction ................................................................... 1 Transcatheter Endovascular Revascularization— CPT Codes and Descriptions ..........................................................1 Femoral/Popliteal Vascular Territory .......................... 123 Procedure Codes ..............................................................................3 Transcatheter Endovascular Chapter 1: The Basics ...................................................... 7 Revascularization—Tibial/Peroneal Vascular APC Basics–Why Is This Important? .............................................7 Territory ............................................................................. 126 CCI Edits–Why Is This Important? .............................................. 10 Endovascular Transluminal Angioplasty— Visceral and Recovery Audit Contractors (RAC) ............................................ 11 Brachiocephalic Arteries, Aorta, and the Coding Basics ................................................................................. 11 Venous System ................................................................. 129 General Coding Guidelines ......................................................... 12 Transluminal Atherectomy for Supra-Inguinal Arteries .... 136 Modifiers for -

Peripheral Artery Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic)
ACCF/AHA Pocket Guideline November 2011 Management of Patients With Peripheral Artery Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) Adapted from the 2005 ACCF/AHA Guideline and the 2011 ACCF/AHA Focused Update Developed in Collaboration With the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery © 2011 by the American College of Cardiology Foundation and the American Heart Association, Inc. The following material was adapted from the 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease J Am Coll Cardiol 2011; 58:2020-2045 and the 2005 ACC/AHA guidelines for the management of the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) J Am Coll Cardiol 2006;47:1239-312. This pocket guideline is available on the World Wide Web sites of the American College of Cardiology (cardiosource.org) and the American Heart Association (my.americanheart.org). For copies of this document, please contact Elsevier Inc. Reprint Department, e-mail: [email protected]; phone: 212-633-3813; fax: 212-633-3820. Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American College of Cardiology Foundation. Please contact Elsevier’s permission department at [email protected]. B Contents 1. Introduction -

82229658.Pdf
Comparison of intravascular ultrasonography and intraarterial digital subtraction angiography after directional atherectomy of short lesions in femoropopliteal arteries Alexander V. Tielbeek, MD, Dammis Vroegindeweij, MD, PhD, Jacob Buth, MD, PhD, Francois P. G. Schol, MD, PhD, and WiUem P. Th. M. Mali, MD, PhD, Eindhoven and Utrecht, The Netherlands Purpose: In this study a group of patients undergoing directional atherectomy for localized occlusive disease in the femoropopliteal arteries, the value of intravascular ultrasonogra- phy (IVUS) to improve the efficacy of plaque removal was evaluated. The findings obtained by IVUS were correlated with intraarterial digital subtraction angiography (IA DSA) performed during the procedure. In addition, the patency rates at follow-up in patients undergoing atherectomy with and without IVUS were compared. Methods: Forty patients were treated by atherectomy because of segmental lesions of the femoropopliteal arteries causing intermittent claudication. Twenty-two patients under- went atherectomy, guided by biplane IA DSA only, and 18 patients were also studied by IVUS. The groups were divided by means of consecutive presentation, IVUS being used in the second part of the study period. The median follow-up was 16 months (range, 0 to 40 months). Variables, measured by IVUS during the procedure, were the minimal transverse luminal diameter (MTLD) and the free luminal area. Patency rates at follow-up were determined by regular color-flow duplex examinations. Color-flow duplex criteria for occlusion were absence of arterial flow and, for restenosis, a ratio of peak systolic velocities at the diseased segment and a normal segment of 2.5 or greater. Results: Qualitative IVUS assessment prompted additional atherotome passages because of insufficient atheroma removal or nonaesthetic appearance of the vessel lumen in 15 of the 18 patients who underwent this examination. -

Jetstream Calcium Study
CLINICAL RESEARCH PERIPHERAL INTERVENTIONS Euro Intervention 2015;11: 96-103 Intravascular ultrasound evaluation of JETSTREAM atherectomy removal of superficial calcium in peripheral arteries Akiko Maehara1,2, MD; Gary S. Mintz2, MD; Thomas M. Shimshak3, MD; Joseph J. Ricotta 2nd4, MD, MS; Venkatesh Ramaiah5, MD; Malcolm T. Foster 3rd6, MD; Thomas P. Davis7, MD; William A. Gray1*, MD 1. Columbia University Medical Center, New York, NY, USA; 2. Cardiovascular Research Foundation, New York, NY, USA; 3. Wheaton Franciscan Healthcare, Milwaukee, WI, USA; 4. Emory University, Atlanta, GA, USA; 5. Arizona Heart Institute, Phoenix, AZ, USA; 6. Tennova Turkey Creek Medical Center, Knoxville, TN, USA; 7. St. John Hospital and Medical Center, Detroit, MI, USA KEYWORDS Abstract Aims: Endovascular treatment of calcified femoral-popliteal disease is challenging. We sought to evaluate the mechanism of lumen gain when using the JETSTREAM Atherectomy System to treat calcified peripheral • atherectomy artery lesions. • calcium • intravascular Methods and results: The JETSTREAM Calcium Study was a prospective, single-arm, multicentre study ultrasound (IVUS) to evaluate the JETSTREAM Atherectomy System for severely calcified femoral-popliteal artery lesions, i.e., • peripheral arteries patients with claudication and lesions with superficial calcium >90° and >5 mm in length as determined by intravascular ultrasound (IVUS). The 2.1 mm catheter was used in this study without distal protection. Fifty- five patients underwent angiographic screening: 26 (45%) met IVUS inclusion criteria. Angiographic calcium was moderate in eight cases and severe in 14, with no available data for four cases. Visual diameter steno- sis was 86±9% pre-treatment, 37±13% post atherectomy, and 10±6% post adjunctive treatment (adjunctive PTA+stenting in eight and adjunct PTA alone in 16). -

SJH Procedures
SJH Procedures - Cardiac and Cardiovascular Services New Name Old Name CPT Code Service ABLATION AP Cardiovascular, Cardiac ABLATION, ARRHYTHMOGENIC FOCUS, FOR ATRIAL FIBRILLATION ABLATION A-FIB *33250 Operative ablation of supraventricular arrhythmogenic focus Cardiovascular, Cardiac or pathway (eg, Wolff-Parkinson-White, atrioventricular node re-entry), tract(s) and/or focus (foci); without cardiopulmonary bypass *33251 Operative ablation of supraventricular arrhythmogenic focus Cardiovascular, Cardiac or pathway (eg, Wolff-Parkinson-White, atrioventricular node re-entry), tract(s) and/or focus (foci); with cardiopulmonary bypass ABLATION, ARRHYTHMOGENIC FOCUS, FOR ATRIAL FLUTTER ABLATION A-FLUTTER *33250 Operative ablation of supraventricular arrhythmogenic focus Cardiovascular, Cardiac or pathway (eg, Wolff-Parkinson-White, atrioventricular node re-entry), tract(s) and/or focus (foci); without cardiopulmonary bypass *33251 Operative ablation of supraventricular arrhythmogenic focus Cardiovascular, Cardiac or pathway (eg, Wolff-Parkinson-White, atrioventricular node re-entry), tract(s) and/or focus (foci); with cardiopulmonary bypass ABLATION, ARRHYTHMOGENIC FOCUS, FOR ATRIAL TACHYCARDIA ABLATION ATRIAL TACHYCARDIA *33250 Operative ablation of supraventricular arrhythmogenic focus Cardiovascular, Cardiac or pathway (eg, Wolff-Parkinson-White, atrioventricular node re-entry), tract(s) and/or focus (foci); without cardiopulmonary bypass *33251 Operative ablation of supraventricular arrhythmogenic focus Cardiovascular, Cardiac or pathway