Chronic Hypoxia and Rat Lung Development: Analysis by Morphometry and Directed Microarray
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FLNC Missense Variants in Familial Noncompaction Cardiomyopathy
Cardiogenetics 2019; volume 9:8181 FLNC missense variants than 2 according to current echocardio- in familial noncompaction graphic criteria, or 2.3 on CMR.1,2 Correspondence: Jaap I. van Waning, Approximately 10% of patients diagnosed Department of Clinical Genetics, EE 2038, cardiomyopathy with NCCM have concurrent congenital Erasmus MC, POB 2040, 3000CA Rotterdam, heart defects (CHD).3,4 the Netherlands. Tel.: +3107038388 - Fax: +3107043072. Jaap I. van Waning,1 In 30-40% of cases diagnosed with E-mail: [email protected] Yvonne M. Hoedemaekers,2 NCCM a pathogenic variant can be identi- 2,3 4 Wouter P. te Rijdt, Arne I. Jpma, fied. Around 80% of these pathogenic vari- Acknowledgements: JVW was supported by a Daphne Heijsman,4 Kadir Caliskan,5 ants involve the same sarcomere genes, that grant from the Jaap Schouten Foundation. Elke S. Hoendermis,6 are the major causes for hypertrophic car- WPTR was supported by a Young Talent Program (CVON PREDICT) grant 2017T001 Tineke P. Willems,7 diomyopathy (HCM) and dilated cardiomy- - Dutch Heart Foundation. 8 opathy (DCM), in particular MYH7, Arthur van den Wijngaard, 5,6 3 MYBPC3 and TTN. Filamin C (FLNC) Albert Suurmeijer, Conflict of interest: the authors declare no plays a central role in muscle functioning Marjon A. van Slegtenhorst,1 potential conflict of interest. by maintaining the structural integrity of the Jan D.H. Jongbloed,2 muscle fibers. Pathogenic variants in FLNC Received for publication: 20 March 2019. Danielle F. Majoor-Krakauer,1 2 were found to be associated with a wide Revision received: 29 July 2019. Paul A. -

Strategies to Increase ß-Cell Mass Expansion
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ Strategies to increase -cell mass expansion Drynda, Robert Lech Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 Strategies to increase β-cell mass expansion A thesis submitted by Robert Drynda For the degree of Doctor of Philosophy from King’s College London Diabetes Research Group Division of Diabetes & Nutritional Sciences Faculty of Life Sciences & Medicine King’s College London 2017 Table of contents Table of contents ................................................................................................. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

The Cytoskeleton in Cell-Autonomous Immunity: Structural Determinants of Host Defence
Mostowy & Shenoy, Nat Rev Immunol, doi:10.1038/nri3877 The cytoskeleton in cell-autonomous immunity: structural determinants of host defence Serge Mostowy and Avinash R. Shenoy Medical Research Council Centre of Molecular Bacteriology and Infection (CMBI), Imperial College London, Armstrong Road, London SW7 2AZ, UK. e‑mails: [email protected] ; [email protected] doi:10.1038/nri3877 Published online 21 August 2015 Abstract Host cells use antimicrobial proteins, pathogen-restrictive compartmentalization and cell death in their defence against intracellular pathogens. Recent work has revealed that four components of the cytoskeleton — actin, microtubules, intermediate filaments and septins, which are well known for their roles in cell division, shape and movement — have important functions in innate immunity and cellular self-defence. Investigations using cellular and animal models have shown that these cytoskeletal proteins are crucial for sensing bacteria and for mobilizing effector mechanisms to eliminate them. In this Review, we highlight the emerging roles of the cytoskeleton as a structural determinant of cell-autonomous host defence. 1 Mostowy & Shenoy, Nat Rev Immunol, doi:10.1038/nri3877 Cell-autonomous immunity, which is defined as the ability of a host cell to eliminate an invasive infectious agent, is a first line of defence against microbial pathogens 1 . It relies on antimicrobial proteins, specialized degradative compartments and programmed host cell death 1–3 . Cell- autonomous immunity is mediated by tiered innate immune signalling networks that sense microbial pathogens and stimulate downstream pathogen elimination programmes. Recent studies on host– microorganism interactions show that components of the host cell cytoskeleton are integral to the detection of bacterial pathogens as well as to the mobilization of antibacterial responses (FIG. -

From Inverse Agonism to 'Paradoxical Pharmacology' Richard A
International Congress Series 1249 (2003) 27-37 From inverse agonism to 'Paradoxical Pharmacology' Richard A. Bond*, Kenda L.J. Evans, Zsirzsanna Callaerts-Vegh Department of Pharmacological and Pharmaceutical Sciences, University of Houston, 521 Science and Research Bldg 2, 4800 Caltioun, Houston, TX 77204-5037, USA Received 16 April 2003; accepted 16 April 2003 Abstract The constitutive or spontaneous activity of G protein-coupled receptors (GPCRs) and compounds acting as inverse agonists is a recent but well-established phenomenon. Dozens of receptor subtypes for numerous neurotransmitters and hormones have been shown to posses this property. However, do to the apparently low percentage of receptors in the spontaneously active state, the physiologic relevance of these findings remains questionable. The possibility that the reciprocal nature of the effects of agonists and inverse agonists may extend to cellular signaling is discussed, and that this may account for the beneficial effects of certain p-adrenoceptor inverse agonists in the treatment of heart failure. © 2003 Elsevier Science B.V. All rights reserved. Keywords. Inverse agonism; GPCR; Paradoxical pharmacology 1. Brief history of inverse agonism at G protein-coupled receptors For approximately three-quarters of a century, ligands that interacted with G protein- coupled receptors (GPCRs) were classified either as agonists or antagonists. Receptors were thought to exist in a single quiescent state that could only induce cellular signaling upon agonist binding to the receptor to produce an activated state of the receptor. In this model, antagonists had no cellular signaling ability on their own, but did bind to the receptor and prevented agonists from being able to bind and activate the receptor. -
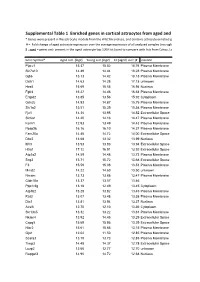
Supplemental Table 1 Enriched Genes in Cortical Astrocytes from Aged
Supplemental Table 1 Enriched genes in cortical astrocytes from aged and young-adult mice * Genes were present in the astrocyte module from the WGCNA analysis, and contains astrocyte enriched genes compared to microglia and oligodendrocytes # = Fold change of aged astrocyte expression over the average expression of all analyzed samples (microglia, astrocytes: young, old, with and without myelin contamination) $ ; aged = genes only present in the aged astrocyte top 1000 list (used to compare with lists from Cahoy, Lovatt, Doyle; see Fig. 4B), all = genes present in all astrocyte top 1000 lists Gene Symbol* Aged astr. (log2) Young astr.(log2) FC (aged/ aver.)# Location Ptprz1 15.37 15.02 18.76 Plasma Membrane Slc7a10 14.49 14.44 18.28 Plasma Membrane Gjb6 15.13 14.42 18.18 Plasma Membrane Dclk1 14.63 14.28 17.18 unknown Hes5 15.69 15.55 16.94 Nucleus Fgfr3 15.27 14.46 16.54 Plasma Membrane Entpd2 13.85 13.56 15.92 Cytoplasm Grin2c 14.93 14.87 15.75 Plasma Membrane Slc1a2 15.51 15.39 15.58 Plasma Membrane Fjx1 14.36 13.98 14.52 Extracellular Space Slc6a1 14.20 14.16 14.47 Plasma Membrane Kcnk1 12.93 13.49 14.43 Plasma Membrane Ppap2b 16.16 16.10 14.37 Plasma Membrane Fam20a 14.48 14.72 14.00 Extracellular Space Dbx2 13.68 13.32 13.99 Nucleus Itih3 13.93 13.93 13.94 Extracellular Space Htra1 17.12 16.91 13.92 Extracellular Space Atp1a2 14.59 14.48 13.73 Plasma Membrane Scg3 15.71 15.72 13.68 Extracellular Space F3 15.59 15.08 13.51 Plasma Membrane Mmd2 14.22 14.60 13.50 unknown Nrcam 13.73 13.88 13.47 Plasma Membrane Cldn10a 13.37 13.57 13.46 -

Genetic Mutations and Mechanisms in Dilated Cardiomyopathy
Genetic mutations and mechanisms in dilated cardiomyopathy Elizabeth M. McNally, … , Jessica R. Golbus, Megan J. Puckelwartz J Clin Invest. 2013;123(1):19-26. https://doi.org/10.1172/JCI62862. Review Series Genetic mutations account for a significant percentage of cardiomyopathies, which are a leading cause of congestive heart failure. In hypertrophic cardiomyopathy (HCM), cardiac output is limited by the thickened myocardium through impaired filling and outflow. Mutations in the genes encoding the thick filament components myosin heavy chain and myosin binding protein C (MYH7 and MYBPC3) together explain 75% of inherited HCMs, leading to the observation that HCM is a disease of the sarcomere. Many mutations are “private” or rare variants, often unique to families. In contrast, dilated cardiomyopathy (DCM) is far more genetically heterogeneous, with mutations in genes encoding cytoskeletal, nucleoskeletal, mitochondrial, and calcium-handling proteins. DCM is characterized by enlarged ventricular dimensions and impaired systolic and diastolic function. Private mutations account for most DCMs, with few hotspots or recurring mutations. More than 50 single genes are linked to inherited DCM, including many genes that also link to HCM. Relatively few clinical clues guide the diagnosis of inherited DCM, but emerging evidence supports the use of genetic testing to identify those patients at risk for faster disease progression, congestive heart failure, and arrhythmia. Find the latest version: https://jci.me/62862/pdf Review series Genetic mutations and mechanisms in dilated cardiomyopathy Elizabeth M. McNally, Jessica R. Golbus, and Megan J. Puckelwartz Department of Human Genetics, University of Chicago, Chicago, Illinois, USA. Genetic mutations account for a significant percentage of cardiomyopathies, which are a leading cause of conges- tive heart failure. -

Bradykinin Receptor Deficiency Or Antagonism Do Not Impact the Host
Ding et al. Intensive Care Medicine Experimental (2019) 7:14 Intensive Care Medicine https://doi.org/10.1186/s40635-019-0228-3 Experimental RESEARCH Open Access Bradykinin receptor deficiency or antagonism do not impact the host response during gram-negative pneumonia-derived sepsis Chao Ding1,2, Jack Yang2, Cornelis van’t Veer2 and Tom van der Poll2,3* * Correspondence: t.vanderpoll@ amc.uva.nl Abstract 2Center of Experimental and Molecular Medicine, Academic Background: Kinins are short peptides with a wide range of proinflammatory Medical Center, University of properties that are generated from kininogens in the so-called kallikrein-kinin system. Amsterdam, Meibergdreef 9, Room Kinins exert their biological activities through stimulation of two distinct receptor G2-130, 1105 AZ Amsterdam, the Netherlands subtypes, the kinin or bradykinin B1 and B2 receptors (B1R, B2R). Acute challenge 3Division of Infectious Diseases, models have implicated B1R and B2R in the pathogenesis of sepsis. However, their Academic Medical Center, role in the host response during sepsis originating from the lung is not known. University of Amsterdam, Amsterdam, the Netherlands Results: To determine the role of B1R and B2R in pneumonia-derived sepsis, B1R/ Full list of author information is B2R-deficient mice and wild-type mice treated with the B1R antagonist R-715 or the available at the end of the article B2R antagonist HOE-140 were studied after infection with the common gram- negative pathogen Klebsiella pneumoniae via the airways. Neither B1R/B2R deficiency nor B1R or B2R inhibition influenced bacterial growth at the primary site of infection or dissemination to distant body sites. -

An Explanation of G-Protein Coupled Receptor Structure
Journal of Stem Cell Research & Therapeutics Review Article Open Access Role of GPCRS towards cell: an explanation of g-protein coupled receptor structure Abstract Volume 2 Issue 3 - 2017 G- protein coupled receptors are the heptahelical, serpentine receptor and are Nidhi Sharma,1 Anil Kumar Sahdev,2 Vinit Raj2 multifunctional receptors having modulatory activity of a wide variety of biological 1Dr. K. N. Modi Institute of Pharmacy & Research centre, process including: Neurotransmission, Chemoattraction, Cardiac function, Olfaction Modinagar, India. and Vision etc. At largest level they are involved in signal transduction across cell 2Department of Pharmaceutical Sciences, Babasaheb Bhimrao membranes therefore they represent major targets in the development of novel drug Ambedkar University, India candidates in all clinical areas. Particularly membrane cholesterol has been reported to have a great role in the functioning of a number of GPCRs. Apart from this it also Correspondence: Vinit Raj, Department of Pharmaceutical have some drawbacks. Mutations that occur are associated with a broad spectrum of Sciences, Babasaheb Bhimrao Ambedkar University, VidyaVihar, diseases of diverse etiology. As a mutations result, there is a change in receptor activity Rae Bareli Road, Lucknow-226025, Email [email protected] (GPCR become inactive, overactive, or constitutively active), in the process of ligand binding and signal transduction. Changes in the GPCRs functioning can cause diseases Received: January 31, 2017 | Published: March 27, 2017 such as retinitis pigmentosa (rhodopsin mutations), nephrogenic diabetes insipidus (vasopressin receptor mutations), and obesity (melanocortin receptor mutations). Keywords: gpcrs, serpentine receptor, neurotransmission, retinitis pigmentosa, nephrogenic diabetes insipidus, bardet-biedl syndrome, hypogonadism Introduction deliver a message and appropriate cellular and physiological response1,2 (Figure 1). -

Panoply™ Human NPR3 Over-Expressing Stable Cell Line
Panoply™ Human NPR3 Over-expressing Stable Cell Line HEK293-NPR3 Lot. No. (See product label) CELL LINE INFORMATION Cat.No. CSC-SC010567 Cell Line Description HEK293-NPR3 cell line is clonally derived from HEK293 cell line, which has been transduced with a lentiviral vector encoding: 1) full length human NPR3 under the CMV promoter, and 2) puromycin resistance gene under the control of PGK promoter. Overexpression of human NPR3 has been quantified by qPCR. Background NPR3 (natriuretic peptide receptor 3) is an atrial natriuretic peptide receptor that in humans is encoded by the NPR3 gene. This protein is one of three natriuretic peptide receptors. It is responsible for clearing circulating and extracellular natriuretic peptides through endocytosis of the receptor. Natriutetic peptides are small peptides which regulate blood volume and pressure, pulmonary hypertension, and cardiac function as well as some metabolic and growth processes. Host Cell HEK293 Gene Information Gene Name Natriuretic peptide receptor 3 Official Symbol NPR3 Species Homo sapiens (human) GeneID 4883 mRNA Reference NM_000908.3 Protein Reference NP_000899.1 Synonyms NPRC; ANP-C; ANPRC; NPR-C; ANPR-C; GUCY2B; C5orf23 SAFETY AND PACKAGING Mycoplasma Mycoplasma Status: Negative (MycoAlert Kit) Format One frozen vial Storage Liquid nitrogen Safety Considerations The following safety precautions should be observed. 1. Use pipette aids to prevent ingestion and keep aerosols down to a minimum. 2. No eating, drinking or smoking while handling the stable line. 3. Wash hands after handling the stable line and before leaving the lab. 4. Decontaminate work surface with disinfectant or 70% ethanol before and after working with stable cells. -

Investigation of Candidate Genes and Mechanisms Underlying Obesity
Prashanth et al. BMC Endocrine Disorders (2021) 21:80 https://doi.org/10.1186/s12902-021-00718-5 RESEARCH ARTICLE Open Access Investigation of candidate genes and mechanisms underlying obesity associated type 2 diabetes mellitus using bioinformatics analysis and screening of small drug molecules G. Prashanth1 , Basavaraj Vastrad2 , Anandkumar Tengli3 , Chanabasayya Vastrad4* and Iranna Kotturshetti5 Abstract Background: Obesity associated type 2 diabetes mellitus is a metabolic disorder ; however, the etiology of obesity associated type 2 diabetes mellitus remains largely unknown. There is an urgent need to further broaden the understanding of the molecular mechanism associated in obesity associated type 2 diabetes mellitus. Methods: To screen the differentially expressed genes (DEGs) that might play essential roles in obesity associated type 2 diabetes mellitus, the publicly available expression profiling by high throughput sequencing data (GSE143319) was downloaded and screened for DEGs. Then, Gene Ontology (GO) and REACTOME pathway enrichment analysis were performed. The protein - protein interaction network, miRNA - target genes regulatory network and TF-target gene regulatory network were constructed and analyzed for identification of hub and target genes. The hub genes were validated by receiver operating characteristic (ROC) curve analysis and RT- PCR analysis. Finally, a molecular docking study was performed on over expressed proteins to predict the target small drug molecules. Results: A total of 820 DEGs were identified between -

Flow Reagents Single Color Antibodies CD Chart
CD CHART CD N° Alternative Name CD N° Alternative Name CD N° Alternative Name Beckman Coulter Clone Beckman Coulter Clone Beckman Coulter Clone T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells CD1a T6, R4, HTA1 Act p n n p n n S l CD99 MIC2 gene product, E2 p p p CD223 LAG-3 (Lymphocyte activation gene 3) Act n Act p n CD1b R1 Act p n n p n n S CD99R restricted CD99 p p CD224 GGT (γ-glutamyl transferase) p p p p p p CD1c R7, M241 Act S n n p n n S l CD100 SEMA4D (semaphorin 4D) p Low p p p n n CD225 Leu13, interferon induced transmembrane protein 1 (IFITM1). p p p p p CD1d R3 Act S n n Low n n S Intest CD101 V7, P126 Act n p n p n n p CD226 DNAM-1, PTA-1 Act n Act Act Act n p n CD1e R2 n n n n S CD102 ICAM-2 (intercellular adhesion molecule-2) p p n p Folli p CD227 MUC1, mucin 1, episialin, PUM, PEM, EMA, DF3, H23 Act p CD2 T11; Tp50; sheep red blood cell (SRBC) receptor; LFA-2 p S n p n n l CD103 HML-1 (human mucosal lymphocytes antigen 1), integrin aE chain S n n n n n n n l CD228 Melanotransferrin (MT), p97 p p CD3 T3, CD3 complex p n n n n n n n n n l CD104 integrin b4 chain; TSP-1180 n n n n n n n p p CD229 Ly9, T-lymphocyte surface antigen p p n p n