Redalyc.Plasticity in Feeding Selectivity and Trophic Structure of Kelp Forest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FIP N° 2003-22 CARACTERIZACIÓN ECOLÓGICA Y PESQUERA DEL ÁREA DE RESERVA ARTESANAL ENTRE LA I Y II REGIONES
INFORME FINAL FIP N° 2003-22 Caracterización ecológica y pesquera del área de reserva artesanal entre la I y II Regiones • Junio, 2005 • REQUIRENTE FONDO DE INVESTIGACIÓN PESQUERA, FIP Presidente del Consejo: Felipe Sandoval Precht EJECUTOR INSTITUTO DE FOMENTO PESQUERO, IFOP Jefe División Investigación Pesquera: Mauricio Braun Alegría Director Ejecutivo: Guillermo Moreno Paredes • Junio, 2005 • JEFE DE PROYECTO JORGE GONZÁLEZ YÁÑEZ. AUTORES JORGE GONZÁLEZ Y. MÓNICA CATRILAO C. NANCY BARAHONA T. CARLOS MARTÍNEZ F. CARLOS TAPIA J. ÁLVARO WILSON M. JORGE GARRIDO P. VÍCTOR BAROS P. ZAIDA YOUNG U. CARLOS CORTES S. CÉSAR GUEVARA P. CARLOS GASPAR S. JUAN SAAVEDRA N. • Junio, 2005 • INSTITUTO DE FOMENTO PESQUERO / DIVISIÓN DE INVESTIGACIÓN PESQUERA RESUMEN EJECUTIVO El sector pesquero de la I y II Regiones, comprende un importante segmento de la economía nacional, contribuyendo fuertemente con el empleo en las zonas costeras y al abastecimiento de materia prima. En esta macrozona la pesca está representada por dos sectores: industrial y artesanal y a su vez está constituida por tres segmentos principales de recursos; pesquerías bentónicas, pesquerías de peces y recolección de algas. Dada la importancia de la actividad extractiva artesanal e industrial en la franja de las 5 millas de la I y II Regiones, en el presente informe se describe y analiza la actividad extractiva de las principales pesquerías. Además, se entrega una completa revisión de los principales antecedentes eco-pesqueros del litoral de las regiones I y II, los cuales en conjunto con la información generada en el marco del proyecto, permitió generar una base de información georreferenciada, actualizada e integrada, para la franja de las 5 millas. -

Fecha: 16 / 05 / 2018 /
Ficha de Claves Gráficas para el Reconocimiento de Peces de Roca Número de ficha: Ficha 02 Fecha: 16 / 05 / 2018 / 1. Especie/ Estado de desarrollo Nombre Común Nombre Científico Rollizo Pinguipes Chilensis (Valenciennes, 1833) Reino Animalia (Kingdom) Clase Actinopterygii (Class) Filo Chordata (Phylum) Orden Perciformes (Order) Subfilo Vertebrata (Subphylum) Suborden Trachinoidei (Suborder) Superclase Gnathostomata (Superclass) Familia Pinguipedidae (Family) Superclase Pisces (Superclass) Género Pinguipes (Genus) Especie Pinguipes chilensis (Species) Fuente: Froese, R. and D. Pauly. Editors. (2018). FishBase. Pinguipes chilensis Valenciennes, 1833. Accessed through: World Register of Marine Species at: http://marinespecies.org/aphia.php?p=taxdetails&id=279407 Fuente: https://www.fishbase.de/summary/12942 2. Distribución Geográfica/Profundidad en la que habita/ Característica del Hábitat Desde Arica hasta Puerto Montt, y Chiloé. En todos lados son iguales, no cambia por lugar. En el sur los encuentras a medio metro. Desde ½ metro hasta 30 metros. (Yo no sé más allá de los 30 metros. Pero no creo que estén más abajo, si abajo no hay comida, la comida está arriba. Las algas llegan a los 25 metros, más abajo no hay. Están en los lugares planos, pero también en los huiros y también se encuevan. 3. Rango de Luminosidad del medio/ En la figura 2, están con flash, aunque estén a 7 metros. 4. Rasgo Sobresaliente sobre el Color del pez respecto al Medio 1 Los puntitos reflejan la luz. La boca amarilla, entre más viejos más amarilla la boca. Se ponen como patos. Es medio gris, como un gris más claro, pero no es morado. Tampoco es plateado. Las castañetas son plateadas. -

Final FIP 2003-22.Pdf
Caracterización ecológica y pesquera del área de reserva artesanal entre la I y II Regiones Item Type Report Authors González, J.; Catrilao, M.; Barahona, N.; Martínez, C.; Tapia, C.; Wilson, A.; Garrido, J.; Baros, V.; Young, Z.; Cortes, C.; Guevara, C.; Gaspar, C.; Saavedra, J. Download date 10/10/2021 21:10:40 Link to Item http://hdl.handle.net/1834/1811 INFORME FINAL FIP N° 2003-22 Caracterización ecológica y pesquera del área de reserva artesanal entre la I y II Regiones • Junio, 2005 • REQUIRENTE FONDO DE INVESTIGACIÓN PESQUERA, FIP Presidente del Consejo: Felipe Sandoval Precht EJECUTOR INSTITUTO DE FOMENTO PESQUERO, IFOP Jefe División Investigación Pesquera: Mauricio Braun Alegría Director Ejecutivo: Guillermo Moreno Paredes • Junio, 2005 • JEFE DE PROYECTO JORGE GONZÁLEZ YÁÑEZ. AUTORES JORGE GONZÁLEZ Y. MÓNICA CATRILAO C. NANCY BARAHONA T. CARLOS MARTÍNEZ F. CARLOS TAPIA J. ÁLVARO WILSON M. JORGE GARRIDO P. VÍCTOR BAROS P. ZAIDA YOUNG U. CARLOS CORTES S. CÉSAR GUEVARA P. CARLOS GASPAR S. JUAN SAAVEDRA N. • Junio, 2005 • INSTITUTO DE FOMENTO PESQUERO / DIVISIÓN DE INVESTIGACIÓN PESQUERA RESUMEN EJECUTIVO El sector pesquero de la I y II Regiones, comprende un importante segmento de la economía nacional, contribuyendo fuertemente con el empleo en las zonas costeras y al abastecimiento de materia prima. En esta macrozona la pesca está representada por dos sectores: industrial y artesanal y a su vez está constituida por tres segmentos principales de recursos; pesquerías bentónicas, pesquerías de peces y recolección de algas. Dada la importancia de la actividad extractiva artesanal e industrial en la franja de las 5 millas de la I y II Regiones, en el presente informe se describe y analiza la actividad extractiva de las principales pesquerías. -

Guía De Peces Litorales
PECES LITORALES de Chile Guía para una pesca recreativa marina sustentable Conservar las GUÍA PARA UNA PESCA RECREATIVA MARINA SUSTENTABLE tierras y aguas de las cuales depende la vida. Publicación realizada por The Nature Conservancy Este trabajo es producto de la colaboración de expertos académicos, de instituciones del estado, federaciones de pesca recreativa y ONGs. Santiago de Chile, 2020 Nature.org/LatinAmerica nature_org nature_org thenatureconservancy - 3 - GUÍA PARA UNA PESCA RECREATIVA MARINA SUSTENTABLE La pesca recreativa una actividad sustentable The Nature Conservancy (TNC) es una Organización No de Chile. Este grupo de especies incluyen especies carnívoras Gubernamental (ONG) con la misión de conservar y proteger y micro-carnívoras tales como la Vieja Negra o Mulato (Graus las aguas y tierras de las que depende la vida en las princi- nigra), Pejeperro (Semicossyphus darwini), Apañao (Hemilut- pales regiones del planeta, en línea con el desarrollo social, janus macrophthalmos) y Bilagay o Pintacha (Cheilodactylus económico y el respeto por las comunidades. En Chile y Perú, variegatus); omnívoros como el Acha (Medialuna ancietae) y el estamos trabajando en proteger y dar un uso sustentable a Baunco (Girella laevifrons), y el herbívoro Jerguilla (Aplodactylus los recursos pesqueros que son parte del ecosistema mari- punctatus), entre otros. Las especies más emblemáticas del no de la Corriente de Humboldt, especialmente de los peces litoral chileno son tres; la Vieja Negra o Mulato, el Pejeperro litorales y del hábitat marino costero que generan las algas y el Acha y son las que muestran los mayores problemas de pardas. conservación, debido a que alcanzan grandes tamaños y va- lor comercial. -

Redalyc.Revisión Bibliográfica De Especies Ectoparásitas Y
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Muñoz, Gabriela; Olmos, Viviana Revisión bibliográfica de especies ectoparásitas y hospedadoras de sistemas acuáticos de Chile Revista de Biología Marina y Oceanografía, vol. 42, núm. 2, agosto, 2007, pp. 89-148 Universidad de Valparaíso Viña del Mar, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=47942201 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Revista de Biología Marina y Oceanografía 42(2): 89 – 148, agosto de 2007 Revisión bibliográfica de especies ectoparásitas y hospedadoras de sistemas acuáticos de Chile Bibliographic revision of ectoparasite and host species from aquatic systems of Chile Gabriela Muñoz1 y Viviana Olmos2 1Facultad de Ciencias del Mar y de Recursos Naturales, Universidad de Valparaíso, Casilla 5080, Reñaca, Viña del Mar, Chile 2Departamento de Zoología, Facultad de Ciencias, Universidad de Concepción, Casilla 160-C, Concepción, Chile [email protected] Abstract.- To date there are about 380 scientific Resumen.- Actualmente existen cerca de 380 publications on eumetazoan parasites collected from aquatic publicaciones científicas referidas a parásitos eumetazoos animals from Chile. However, all this information is spread obtenidos de animales acuáticos recolectados a lo largo de on different bibliographic sources including some old articles Chile. Sin embargo, esta literatura está dispersa en distintas (a century old). Few studies have summarized in checklists fuentes bibliográficas; algunas con más de un siglo de the surveys of some groups of parasite and host species. -

Actualización Y Validación De La Clasificación De Las Zonas Biogeográficas Litorales
Proyecto FIP N° 2004 – 28 INFORME FINAL ACTUALIZACIÓN Y VALIDACIÓN DE LA CLASIFICACIÓN DE LAS ZONAS BIOGEOGRÁFICAS LITORALES UNIVERSIDAD AUSTRAL DE CHILE Enero de 2006 RESUMEN EJECUTIVO El objetivo general de este Proyecto fue actualizar la clasificación de las zonas zoogeográficas litorales de la costa de Chile, determinando los factores físicos y biológicos que permiten caracterizar esas zonas. Se analizaron bases de datos de las características físicas de la costa de Chile, incluyendo rugosidad de la costa, ancho de la plataforma continental, vientos costeros, temperatura superficial del mar, radiación solar y concentración (satelital) de clorofila y de la distribución y abundancia de invertebrados y peces de ambientes inter y submareales y de fondos blandos y rocosos y base de datos de macroalgas. a) Características físicas y ambientales de la costa de Chile La costa de Chile puede ser dividida en tres grandes zonas de acuerdo a la rugosidad de la misma: (i) una costa extremadamente rugosa entre 51 y 55ºS, (ii) una costa rugosa entre 46 y 50ºS y (iii) una costa poco rugosa desde 40ºS hacia el norte. Cuatro zonas se pueden reconocer en base al ancho de la plataforma: (i) plataforma muy angosta (< 10 km) entre el límite norte y 33,5ºS; (ii) plataforma menos angosta (>10 km, < 20 km) entre 33,5ºS y 36ºS; (iii) plataforma ancha (hasta 70 km) entre 36ºS hasta 40ºS; (iv) ancho dificilmente definible al sur de 40ºS, por la extrema rugosidad de la zona. Desde el punto de vista de los vientos, se tienen al menos 3 zonas geográficas: (i) la zona centro- norte del país, desde el límite norte del país hasta 39ºS, con vientos meridionales hacia el norte durante todo el año; (ii) una zona de transición entre 39 y 45ºS, en que la dirección de los vientos meridionales depende de la estación del año; e (iii) la zona austral del país, al sur de 45ºS, en que los vientos meridionales soplan hacia el sur durante todo el año. -
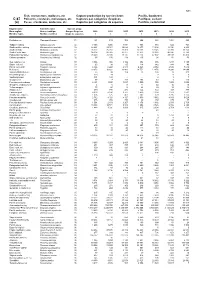
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
531 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2009 2010 2011 2012 2013 2014 2015 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 481 613 302 806 323 1 804 483 Tadpole codling Salilota australis 32 3 356 1 400 1 091 768 374 522 703 Southern blue whiting Micromesistius australis 32 22 221 23 301 19 629 16 675 15 304 10 036 8 809 Southern hake Merluccius australis 32 26 272 25 361 20 909 20 288 19 346 12 393 16 150 South Pacific hake Merluccius gayi 32 94 306 90 305 82 977 72 872 92 031 96 196 77 283 Patagonian grenadier Macruronus magellanicus 32 78 440 74 330 70 137 62 175 47 602 39 138 37 475 Chilean grenadier Coelorinchus chilensis 32 65 156 134 136 91 54 59 Sea catfishes nei Ariidae 33 3 096 406 1 426 852 876 1 217 1 185 Snake eels nei Ophichthidae 33 60 38 65 114 142 144 49 Pacific cornetfish Fistularia corneta 33 6 396 6 443 4 513 4 323 4 854 2 534 7 630 Mullets nei Mugilidae 33 18 673 10 821 13 400 18 751 13 876 14 290 14 044 Snooks(=Robalos) nei Centropomus spp 33 25 98 104 78 136 79 310 Broomtail grouper Mycteroperca xenarcha 33 120 14 .. -

Historia Física Y Política De Chile
21 Historia física Claudio Gay y política de Chile (1800-1873) La Biblioteca fundamentos de la construcción de Chile En los inicios de la república, cuando todo estaba El naturalista francés arribó a Chile en es una iniciativa de la Cámara Chilena de por hacerse, cuando Chile sólo existía como proyecto Zoología II 1828 para trabajar como profesor. En 1829, la Construcción, en conjunto con la Pon- institucional, ¿cómo era el territorio bajo la jurisdicción inició su labor de reconocimiento del terri- tificia Universidad Católica de Chile y la del nuevo Estado?, ¿cuáles, las características físicas, torio nacional. Un año después comenzó su Claudio Gay Dirección de Bibliotecas, Archivos y Museos económicas, culturales y sociales del conjunto bajo su trabajo más importante al suscribir un con- que, en formato impreso y multimedia, soberanía?, ¿cuál, la noción existente acerca del número Claudio Gay trato con el gobierno. En éste, el sabio se reúne las obras de los científicos, profesio- y distribución espacial de sus habitantes?, ¿cuáles, sus comprometió a hacer un viaje científico por nales y técnicos que con sus trabajos dieron principales recursos económicos?, ¿cuáles, sus carac- todo el territorio con el objeto de investigar a conocer Chile –sus recursos humanos y terísticas ambientales?, ¿sus potencialidades? A éstas, la historia natural de Chile y todo aquello naturales, así como sus características sociales y muchas otras interrogantes buscaba dar respuestas el que contribuyera a dar a conocer sus produc- y evolución histórica– contribuyendo con gobierno chileno cuando en 1830 decidió la contrata- tos. sus investigaciones, informes y trabajos a la ción de Claudio Gay. -

Informe Final Proyecto Fip 2006-56
INFORME FINAL PROYECTO FIP 2006-56 EVALUACION DE LINEA BASE DE LAS RESERVAS MARINAS “ISLA CHAÑARAL” E “ISLA CHOROS- DAMAS” Preparado por Facultad de Ciencias del Mar Universidad Católica del Norte Coquimbo -Agosto 2008- Informe Final Proyecto FIP 2006-56 INDICE INDICE II ÍNDICE DE TABLAS. IV ÍNDICE DE FIGURAS. VI INDICE DE ANEXOS VIII 1.- RESUMEN EJECUTIVO 10 2.- ANTECEDENTES 16 3.- OBJETIVOS 22 4.- METODOLOGÍA 23 4.1.- RECOPILACIÓN DE LA INFORMACIÓN BIBLIOGRÁFICA. 23 4.2.- DESCRIPCIÓN DE LAS PRINCIPALES CARACTERÍSTICAS OCEANOGRÁFICAS 23 4.3.- LEVANTAMIENTO BATIMÉTRICO 27 4.4.- GENERACIÓN DE LAS CARTAS TEMÁTICAS EN SIG 28 4.5.- RIQUEZA, DISTRIBUCIÓN Y ABUNDANCIA DE AVES Y MAMÍFEROS MARINOS 31 4.6.- CARACTERIZACIÓN DE HÁBITATS Y COMUNIDADES INTERMAREALES Y SUBMAREALES 35 4.6.1.- COMUNIDADES INTERMAREALES 36 4.6.2.- COMUNIDADES SUBMAREALES 38 4.7.- EVALUACIONES POBLACIONALES DE RECURSOS OBJETIVOS Y PECES 38 4.7.1.- RECURSOS OBJETIVO 38 4.7.2.- PECES 39 4.8.- ANÁLISIS DEL IMPACTO SOCIAL DE LA RESERVA MARINA. 39 5.- RESULTADOS Y DISCUSIÓN 53 5.1.- RECOPILACIÓN DE LA INFORMACIÓN BIBLIOGRÁFICA. 53 5.2.- DESCRIPCIÓN DE LAS PRINCIPALES CARACTERÍSTICAS OCEANOGRÁFICAS 56 5.3.- LEVANTAMIENTO BATIMÉTRICO 65 5.3.1.- MODELACIÓN TRIDIMENSIONAL DE CARTOGRAFÍA BASE (BATIMETRÍA Y TOPOGRAFÍA) 65 5.3.2.- CARTOGRAFÍA TEMÁTICA 68 5.4.- AVES Y MAMIFEROS MARINOS 68 ii Informe Final Proyecto FIP 2006-56 5.4.1.- ISLA CHOROS 68 5.4.2.- ISLA CHAÑARAL 69 5.4.3.- ISLA DAMAS 70 5.4.4.- ISLAS PÁJAROS 1 Y 2, TILGO, CHUNGUNGO Y TOTORALILLO 70 5.4.5.- COMENTARIOS ACERCA DE ALGUNAS ESPECIES DE AVES MARINAS ENDÉMICAS DEL SISTEMA DE SURGENCIA DE LA CORRIENTE DE HUMBOLDT. -

Feeding Habits and Dietary Overlap During the Larval Development of Two Sandperches (Pisces: Pinguipedidae)
SCIENTIA MARINA 81(2) June 2017, 195-204, Barcelona (Spain) ISSN-L: 0214-8358 doi: http://dx.doi.org/10.3989/scimar.04544.06A Feeding habits and dietary overlap during the larval development of two sandperches (Pisces: Pinguipedidae) Javier A. Vera-Duarte, Mauricio F. Landaeta Laboratorio de Ictioplancton (LABITI), Facultad de Ciencias del Mar y de Recursos Naturales, Universidad de Valparaíso, Avenida Borgoño 16344, Reñaca, Viña del Mar, Chile. (JAV-D) E-mail: [email protected]. ORCID-iD: http://orcid.org/0000-0002-0539-1245 (MFL) (Corresponding author) E-mail: [email protected]. ORCID-iD: http://orcid.org/0000-0002-5199-5103 Summary: Two species of sandperch (Pinguipedidae: Perciformes), Prolatilus jugularis and Pinguipes chilensis, inhabit the coastal waters of the South Pacific. Both species have pelagic larvae with similar morphology, but their diet preferences are unknown. Diet composition, feeding success, trophic niche breadth and dietary overlap were described during larval stages for both species. In the austral spring, larval P. jugularis (3.83-10.80 mm standard length [SL]) and P. chilensis (3.49-7.71 mm SL) during their first month of life had a high feeding incidence (>70%) and fed mostly on copepod nauplii (>80% IRI), Rhincalanus nasutus metanauplii and Paracalanus indicus copepodites. The number of prey ingested was low (mean: 4-5 prey per gut) and independent of larval size; total prey volume and maximum prey width increased as larvae grew. Mouth opening and ingested prey were greater in larval P. jugularis than in P. chilensis, leading to significant differences in prey composition among larval species, in terms of prey number and volume. -

Pinguipedidae
FAMILY Pinguipedidae Gunther, 1860 – sandperches GENUS Kochichthys Kamohara, 1961 - sandperches [=Kochia] Species Kochichthys flavofasciatus (Kamohara, 1936) - Tosa Bay sandperch GENUS Parapercis Bleeker, 1863 - sandperches [=Chilias, Neopercis, Neosillago, Osurus, Parapercichthys, Parapercis S, Percis] Species Parapercis albipinna Randall, 2008 - albipinna sandperch Species Parapercis albiventer Ho et al., 2014 - whitebelly sandperch Species Parapercis alboguttata (Gunther, 1872) - whitespot sandsmelt [=elongata, tesselata] Species Parapercis algrahami Johnson & Worthington Wilmer, 2018 - algrahami sandperch Species Parapercis allporti (Gunther, 1876) - barred grubfish [=ocularis] Species Parapercis altipinnis Ho & Heden, 2017 - altipinnis sandperch Species Parapercis atlantica (Vaillant, 1887) - Atlantic sandperch [=ledanoisi] Species Parapercis aurantiaca Doderlein, in Steindachner & Doderlein, 1884 – aurantiaca sandperch Species Parapercis australis Randall, 2003 - Southern sandperch Species Parapercis banoni Randall & Yamakawa, 2006 - Banon's sandperch Species Parapercis basimaculata Randall et al., 2008 - basimaculata sandperch Species Parapercis bicoloripes Prokofiev, 2010 - bicoloripes sandperch Species Parapercis bimacula Allen & Erdmann, 2012 - redbar sandperch [=pariomaculata] Species Parapercis binivirgata (Waite, 1904) - redbanded weever Species Parapercis binotata Allen & Erdmann, 2017 - binotata sandperch Species Parapercis biordinis Allen, 1976 - biordinis sandperch Species Parapercis caudopellucida Johnson & Motomura, -

Programa Fondecyt Informe Final Etapa 2013
PROGRAMA FONDECYT INFORME FINAL ETAPA 2013 COMISIÓN NACIONAL DE INVESTIGACION CIENTÍFICA Y TECNOLÓGICA VERSION OFICIAL Nº 2 FECHA: 22/05/2014 Nº PROYECTO : 1110235 DURACIÓN : 3 años AÑO ETAPA : 2013 TÍTULO PROYECTO : INFLUENCE OF ENVIRONMENTAL VARIABLES (SALINITY, TEMPERATURE AND DENSITY) ON OSMOREGULATION, ENERGETIC METABOLISM, STRESS RESPONSES AND GROWTH IN THE CHILEAN SEA BASS "ROBALO" ELEGINOPS MACLOVINUS DISCIPLINA PRINCIPAL : ACUICULTURA GRUPO DE ESTUDIO : SALUD PROD ANIM INVESTIGADOR(A) RESPONSABLE : LUIS HUMBERTO VARGAS CHACOFF DIRECCIÓN : COMUNA : CIUDAD : Valdivia REGIÓN : XIV REGION FONDO NACIONAL DE DESARROLLO CIENTIFICO Y TECNOLOGICO (FONDECYT) Moneda 1375, Santiago de Chile - casilla 297-V, Santiago 21 Telefono: 2435 4350 FAX 2365 4435 Email: [email protected] INFORME FINAL PROYECTO FONDECYT REGULAR OBJETIVOS Cumplimiento de los Objetivos planteados en la etapa final, o pendientes de cumplir. Recuerde que en esta sección debe referirse a objetivos desarrollados, NO listar actividades desarrolladas. Nº OBJETIVOS CUMPLIMIENTO FUNDAMENTO 1 “Influence of acclimation to different TOTAL TOTAL 100 % COMPLETADO: environmental salinities on osmoregulation, 4 Productos fueron obtenidos de este objetivo. energetic metabolic and stress response” 1) Como parte de este objetivo se produce la tesis del Sr. Fernando Moneva, la cual se encuentra calificada y la defensa fue realizada en marzo del 2013. 2) Comunicaciones a Congresos: 3) Artículo científico en evaluación en la revista Polar Biology: Environmental salinity modified osmoregulatory response in sub-Antarctic Notothenioid fish Eleginops maclovinus 4) Artículo científico en revisión de los autores y será enviado a la revista Polar Biology, como segunda parte. Metabolic effects like response at saline changes in Sub-Antarctic notothenioid fish Eleginops maclovinus 2 Growth in different environmental salinities TOTAL TOTAL 100 % COMPLETADO 4 Productos fueron obtenidos de este objetivo.