Protamine Newborn Use Only 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent N0.: US 8,343,962 B2 Kisak Et Al
US008343962B2 (12) United States Patent (10) Patent N0.: US 8,343,962 B2 Kisak et al. (45) Date of Patent: *Jan. 1, 2013 (54) TOPICAL FORMULATION (58) Field of Classi?cation Search ............. .. 514/226.5, 514/334, 420, 557, 567 (75) Inventors: Edward T. Kisak, San Diego, CA (US); See application ?le fOr Complete Search history. John M. NeWsam, La Jolla, CA (US); _ Dominic King-Smith, San Diego, CA (56) References C‘ted (US); Pankaj Karande, Troy, NY (US); Samir Mitragotri, Goleta, CA (US) US' PATENT DOCUMENTS 5,602,183 A 2/1997 Martin et al. (73) Assignee: NuvoResearchOntano (CA) Inc., Mississagua, 6,328,979 2B1 12/2001 Yamashita et a1. 7,001,592 B1 2/2006 Traynor et a1. ( * ) Notice: Subject to any disclaimer, the term of this 7,795,309 B2 9/2010 Kisak eta1~ patent is extended or adjusted under 35 2002/0064524 A1 5/2002 Cevc U.S.C. 154(b) by 212 days. FOREIGN PATENT DOCUMENTS This patent is subject to a terminal dis- W0 WO 2005/009510 2/2005 claimer- OTHER PUBLICATIONS (21) APPI' NO‘, 12/848,792 International Search Report issued on Aug. 8, 2008 in application No. PCT/lB2007/0l983 (corresponding to US 7,795,309). _ Notice ofAlloWance issued on Apr. 29, 2010 by the Examiner in US. (22) Med Aug- 2’ 2010 Appl. No. 12/281,561 (US 7,795,309). _ _ _ Of?ce Action issued on Dec. 30, 2009 by the Examiner in US. Appl. (65) Prior Publication Data No, 12/281,561 (Us 7,795,309), Us 2011/0028460 A1 Feb‘ 3’ 2011 Primary Examiner * Raymond Henley, 111 Related U 5 Application Data (74) Attorney, Agent, or Firm * Foley & Lardner LLP (63) Continuation-in-part of application No. -

Streptokinase) and Streptococcal Desoxyribonuclease on Fibrinous, Purulent, and Sanguinous Pleural Exudations
THE EFFECT IN PATIENTS OF STREPTOCOCCAL FIBRINOLYSIN (STREPTOKINASE) AND STREPTOCOCCAL DESOXYRIBONUCLEASE ON FIBRINOUS, PURULENT, AND SANGUINOUS PLEURAL EXUDATIONS William S. Tillett, Sol Sherry J Clin Invest. 1949;28(1):173-190. https://doi.org/10.1172/JCI102046. Research Article Find the latest version: https://jci.me/102046/pdf THE EFFECT IN PATIENTS OF STREPTOCOCCAL FIBRINOLYSIN (STREPTOKINASE) AND STREPTOCOCCAL DESOXYRIBO- NUCLEASE ON FIBRINOUS, PURULENT, AND SAN- GUINOUS PLEURAL EXUDATIONS' By WILLIAM S. TILLETT AND SOL SHERRY (From the Department of Medicine, New York University College of Medicine, and the Third Medical Division of Bellevue Hospital, New York City) (Received for publication August 6, 1948) The results described in this article were ob- coccal groups C and G (3). The product is tained by the injection of concentrated and par- abundantly excreted into the culture medium in tially purified preparations derived from broth which the organisms are grown and is readily ob- cultures of hemolytic streptococci into the pleural tainable free from the bacterial cells in sterile cavity of selected patients who were suffering filtrates. from different types of diseases that gave rise to The fibrinolytic action, in tests conducted un- pleural exudations. The possibility has been ex- der optimal laboratory conditions, is unusually plored of utilizing two of the defined properties rapid in action on the fibrin coagulum of normal elaborated by hemolytic streptococci that have the human blood, requiring only a few minutes when unique capacity of causing rapid lysis of the solid whole plasma is employed as a source of fibrin, elements (fibrin and nucleoprotein) that are sig- and an even shorter time when preparations of nificant parts of exudates. -

Chinese Expert Consensus on Diagnosis and Treatment of Trauma
Song et al. Military Medical Research (2021) 8:25 https://doi.org/10.1186/s40779-021-00317-4 POSITION ARTICLE AND GUIDELINES Open Access Chinese expert consensus on diagnosis and treatment of trauma-induced hypercoagulopathy Jing-Chun Song1* , Li-Kun Yang2, Wei Zhao3, Feng Zhu4, Gang Wang5, Yao-Peng Chen6, Wei-Qin Li7* , Chinese People’s Liberation Army Professional Committee of Critical Care Medicine and Chinese Society of Thrombosis, Hemostasis and Critical Care, Chinese Medicine Education Association Abstract Trauma-induced coagulopathy (TIC) is caused by post-traumatic tissue injury and manifests as hypercoagulability that leads to thromboembolism or hypocoagulability that leads to uncontrollable massive hemorrhage. Previous studies on TIC have mainly focused on hemorrhagic coagulopathy caused by the hypocoagulable phenotype of TIC, while recent studies have found that trauma-induced hypercoagulopathy can occur in as many as 22.2–85.1% of trauma patients, in whom it can increase the risk of thrombotic events and mortality by 2- to 4-fold. Therefore, the Chinese People’s Liberation Army Professional Committee of Critical Care Medicine and the Chinese Society of Thrombosis, Hemostasis and Critical Care, Chinese Medicine Education Association jointly formulated this Chinese Expert Consensus comprising 15 recommendations for the definition, pathophysiological mechanism, assessment, prevention, and treatment of trauma-induced hypercoagulopathy. Keywords: Trauma, Coagulation dysfunction, Thrombosis, Diagnosis, Treatment Background by the hypocoagulable phenotype of TIC [5], while it has re- Trauma causes at least 5.8 million deaths every year in the cently been shown that the incidence of trauma-induced whole world, which account for 9% of annual deaths [1]. -

Correlation of the Production of Plasminogen Activator with Tumor Metastasis in Bi 6 Mouse Melanoma Cell Lines
[CANCER RESEARCH 40, 288-292, February 1980 0008-5472/80/0040-0000$02.00 Correlation of the Production of Plasminogen Activator with Tumor Metastasis in Bi 6 Mouse Melanoma Cell Lines Bosco Shang Wang,2 Gerard A. McLoughlin,3 Jerome P. Richie, and John A. Mannick Deportment of Surgery, Peter Bent Brigham Hospital (B. S. W., G. A. M., J. P. R., J. A. M.j, and Department of Pathology (B. S. W.j, Harvard Medical School, Boston, Massachusetts 02115 ABSTRACT circulating cancer cells were easily detected in patients whose blood had a higher level of FA. The correlation between the production of plasminogen ac Although the FA of a tumor has been suggested as correlat tivator (PA) of tumors and their metastatic potential was stud ing with its metastatic potential, direct and quantitative evi ied. Bi 6 melanoma cells and ‘‘Bi6 mets' ‘cells (harvested from dence to support this hypothesis is lacking. Therefore, we have the pulmonary metastatic nodules of C57BL/6J mice bearing investigated this issue by comparing the FA of the Bi 6 mouse Bi 6 isografts) were examined with respect to their fibrinolytic melanoma and several of its sublines varying in their potential activity (FA) in tissue culture. Bi 6 mets cells had a significantly for forming metastases. higher FA than did Bi 6 cells. Fl (a Bi 6 subline with a lower incidence of metastasis) and Fl 0 (a highly metastatic Bi 6 subline) were also studied. Fl 0 cells produced more FA than MATERIALS AND METHODS did Fi cells. The difference between the FA's of these tumors Tumors. -

Revista Brasileira De Anestesiologia
Rev BrasBras Anestesiol. Anestesiol. 2013;63(1):163–164 2013;63(1):163-166 REVISTA BRASILEIRA DE ANESTESIOLOGIA Offi cial Publication of the Brazilian Society of Anesthesiology www.sba.com.br/rba/index.asp LETTER TO THE EDITOR Precipitation in Gallipoli: Sugammadex / Amiodarone & Sugammadex / Dobutamine & Sugammadex / Protamine Dear Editor, furosemide (10 mg.mL-1), gentamicin (40 mg.mL-1), glyceryl Sugammadex is a modifi ed gamma cyclodextrin 1-3. Cyclodextrins trinitrate (5 mg.mL-1), heparin (1,000 IU.mL-1), hydrocor- are water soluble cyclic oligosaccharides with a lipophilic tisone (250 mg.mL-1), crystallized insulin (100 IU.mL-1), core. Sugammadex has quickly found a place in clinical use Calcium (Calcium Gluconate Monohydrate 225 mg.10 mL-1 for selective antagonism of neuromuscular blockade with ro- + Calcium levulinate dihitrate 572 mg.10 mL-1), ketamine curonium 1-3. Sugammadex quickly encapsulates steroidal neu- (50 mg.mL-1), levobupivacaine (7.5 mg.mL-1), magnesium romuscular blockers, increasing the amount of encapsulated sulphate (1.2 mEq.mL-1), metamizol sodium (0.5 g.mL-1), steroidal neuromuscular blockers in plasma and separating the methylergobasin maleate (0.2 mg.mL-1), metoclopramide blockers from the nicotinic acetylcholine receptors 1-3. (5 mg.mL-1), metoprolol (1 mg.mL-1), morphine (0.01 g.mL-1), Apart from its use with steroidal neuromuscular block- midazolam (5 mg.mL-1), n-acetylcysteine (100 mg.mL-1), ers, it is known that sugammadex interacts with over 40 naloxone (0.4 mg.mL-1), neostigmine (0.5 mg.mL-1), nitroprus- lipophilic, steroidal and non-steroidal drugs. -
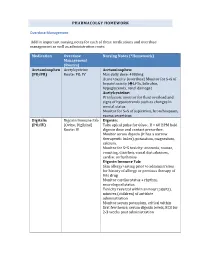
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN
proteomes Article Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN Gianluca Arauz-Garofalo 1, Meritxell Jodar 2,3 , Mar Vilanova 1, Alberto de la Iglesia Rodriguez 2, Judit Castillo 2 , Ada Soler-Ventura 2, Rafael Oliva 2,3,* , Marta Vilaseca 1,* and Marina Gay 1,* 1 Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Baldiri Reixac, 10, 08028 Barcelona, Spain; [email protected] (G.A.-G.); [email protected] (M.V.) 2 Molecular Biology of Reproduction and Development Research Group, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Fundació Clínic per a la Recerca Biomèdica (FCRB), Faculty of Medicine and Health Sciences, University of Barcelona, 08036 Barcelona, Spain; [email protected] (M.J.); [email protected] (A.d.l.I.R.); [email protected] (J.C.); [email protected] (A.S.-V.) 3 Biochemistry and Molecular Genetics Service, Hospital Clínic de Barcelona, 08036 Barcelona, Spain * Correspondence: [email protected] (R.O.); [email protected] (M.V.); [email protected] (M.G.) Abstract: Protamines replace histones as the main nuclear protein in the sperm cells of many species and play a crucial role in compacting the paternal genome. Human spermatozoa contain protamine 1 (P1) and the family of protamine 2 (P2) proteins. Alterations in protamine PTMs or the P1/P2 ratio Citation: Arauz-Garofalo, G.; may be associated with male infertility. Top-down proteomics enables large-scale analysis of intact Jodar, M.; Vilanova, M.; proteoforms derived from alternative splicing, missense or nonsense genetic variants or PTMs. -

Acta Medica Okayama
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Okayama University Scientific Achievement Repository Acta Medica Okayama Volume 23, Issue 5 1969 Article 8 OCTOBER 1969 Various aspects of thrombolysis and fibrinolysis E. Szirmai∗ ∗University of Stuttgart, Copyright c 1999 OKAYAMA UNIVERSITY MEDICAL SCHOOL. All rights reserved. Various aspects of thrombolysis and fibrinolysis∗ E. Szirmai Abstract The author has described modern thrombolytic therapy of arterial and venous thrombosis and emboli by therapeutic fibrinolysis and other drugs also methods and effects of local and par- enteral application of fibrinolysin preparations, dosage, control, indications. Contraindications, side-effects and their treatment with fibrinolysin antagonists and therapy with fibrinolysin com- bined with anticoagulants and antibiotics are discussed. ∗PMID: 4244051 [PubMed - indexed for MEDLINE] Copyright c OKAYAMA UNIVERSITY MEDICAL SCHOOL Szirmai: Various aspects of thrombolysis and fibrinolysis Acta Med. Okayama 23, 429-447 (1969) VARIOUS ASPECTS OF THROMBOLYSIS AND FIBRINOLYSIS E. SZIRMAI Department of Nuclear Hematology and Radiation Biology, In!titute of Nuclear Energy, University of Stuttgart, Stuttgart, Germany Received for publication, August 12, 1.969 There is no essential difference between thrombolysis and fibrinolysis. The use of these two different words in the literature was necessary for practical reasons. We use the expression oC thrombolysis" clinically. The term "fibrinolysis" has been used for the lytic substrates of fibrin in vitro by ASTRUP, GROSS and others. It is better used because the blood clotting thrombus can be dissolved by the lysis of fibrin. The so·called mixed thrombus is composed of cellular elements, particularly containing many platelets. -

Letters to the Editor
Letters to the Editor griseofulvin [ultramicrosize], USP [specially processed) 1 2 S itij$ » t a b l e t s Clinical Considerations: INDICATIONS FULVICIN P/G Tablets are indicated for the treatment of ringworm infections of the skin, hair, and nails, namely: tinea corporis, tinea- pedis, tinea cruris, tinea barbae, tinea capitis, tinea unguium (onychomycosis) when caused by one or more of the following genera of fungi: Trichophyton rubrum. Trichophyton tonsurans. Trichophyton menta- grophytes. Trichophyton mterdigitahs. Trichophyton verrucosum. Trichophyton megnim. Trichophyton gallmae. Trichophyton crateriform. Trichophyton sulph- ureum. Trichophyton schoenlemi. Microsporum audouim. Microsporum canis. Microsporum gypseum. and Epidermophyton floccosum. Note: Prior to therapy, the type of fungi responsible for the infection should be identified. The use of this drug is not justified in minor or trivial infections which will respond to topical agents alone. Griseofulvin is not effective in the following: Bacterial infections. Candidiasis (Moniliasis). Histoplasmosis, Actinomycosis. Sporo trichosis, Chromoblastomycosis. Coccidioidomycosis. North American Blas tomycosis. Cryptococcosis (Torulosis). Tinea versicolor, and Nocardiosis. CONTRAINDICATIONS This drug is contraindicated in patients with porphyria, hepatocellular failure, and in individuals with a history of hypersensitivity to griseofulvin WARNINGS Prophylactic Usage: Safety and efficacy of griseofulvin for prophylaxis of fungal infections have not been established. Animal Toxicology: Chronic feeding of griseofulvin. at levels ranging from 0.5-2.5°o of the diet, resulted in the development of liver tumors in several strains of mice, particularly in males. Smaller particle sizes result in an enhanced effect. Lower oral dosage levels have not been tested. Subcutaneous administration of Treatment of Whiplash Injury relatively small doses of griseofulvin once a week during the f irst three weeks of I would recommend that anyone life has also been reported to induce hepatomata in mice. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Study Protocol
Protocol 3-001 Confidential 28APRIL2017 Version 4.1 Asahi Kasei Pharma America Corporation Synopsis Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Assess the Safety and Efficacy of ART-123 in Subjects with Severe Sepsis and Coagulopathy Name of Sponsor/Company: Asahi Kasei Pharma America Corporation Name of Investigational Product: ART-123 Name of Active Ingredient: thrombomodulin alpha Objectives Primary: x To evaluate whether ART-123, when administered to subjects with bacterial infection complicated by at least one organ dysfunction and coagulopathy, can reduce mortality. x To evaluate the safety of ART-123 in this population. Secondary: x Assessment of the efficacy of ART-123 in resolution of organ dysfunction in this population. x Assessment of anti-drug antibody development in subjects with coagulopathy due to bacterial infection treated with ART-123. Study Center(s): Phase of Development: Global study, up to 350 study centers Phase 3 Study Period: Estimated time of first subject enrollment: 3Q 2012 Estimated time of last subject enrollment: 3Q 2018 Number of Subjects (planned): Approximately 800 randomized subjects. Page 2 of 116 Protocol 3-001 Confidential 28APRIL2017 Version 4.1 Asahi Kasei Pharma America Corporation Diagnosis and Main Criteria for Inclusion of Study Subjects: This study targets critically ill subjects with severe sepsis requiring the level of care that is normally associated with treatment in an intensive care unit (ICU) setting. The inclusion criteria for organ dysfunction and coagulopathy must be met within a 24 hour period. 1. Subjects must be receiving treatment in an ICU or in an acute care setting (e.g., Emergency Room, Recovery Room). -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide