DNA Cleavage Agents from Schisandra Propinqua Var. Sinensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chattahoochee River Pedestrian Bridge: Environmental Assessment
Project No: NA Fulton County P.I. Number 0009640 State Route (SR) 9 at Chattahoochee River in Roswell - Enhancements ENVIRONMENTAL ASSESSMENT U.S. DEPARTMENT OF TRANSPORTATION FEDERAL HIGHWAY ADMINISTRATION AND GEORGIA DEPARTMENT OF TRANSPORTATION SUBMITTED PURSUANT TO 42 USC 4321 et. seq. And 49 USC 303 (for 4(f), if applicable) May 20, 2020 NEPA DATE Eric Duff DATEDAT State Environmental Administrator APPROVAL OF ENVIRONMENTAL ASSESSMENT AND ADVANCEMENT TO AVAILABILITY/PUBLIC HEARING PHASE Digitally signed by JENNIFER L JENNIFER L GIERSCH GIERSCH Date: 2020.06.24 11:59:12 -04'00' DATE FOR: MOISES MARRERO DIVISION ADMINISTRATOR FEDERAL HIGHWAY ADMINISTRATION ǣ͝Ȁ͚͞Ȁ͚͚͘͘ȁǣ ͗ǣ͘͘͘͘͜͡͞ǡǣ ȋ Ȍǣ͝Ȁ͙͙Ȁ͚͙͘͠ 7KHHQJLQHHURIUHFRUG (25 DVVHUWVWKDW ȀǣǤǤ͝Ȁ͚͟Ȁ͚͚͘͘ ǣǤǤ͝Ȁ͚͟Ȁ͚͚͘͘ SODQVLQFRUSRUDWHRUZLOOLQFRUSRUDWHFRPPLWPHQWV 7KH*'27SURMHFWPDQDJHU 30 DVVHUWVWKDW ǣ Ǥ Ǥ͝Ȁ͚͟Ȁ͚͚͘͘ ǣ͝Ȁ͚͞Ȁ͚͚͘͘ WKHVHFRPPLWPHQWVDUHIHDVLEOH LIDSSOLFDEOH ǡ *'2730(ND2NRQPNSDHWRB 6LJQDWXUH'DWH(NDNSDHWR Ǥ (25BBB BBB ǣ͝Ȁ͚͟Ȁ͚͚͘͘ 6LJQDWXUH'DWH Ǥ Ȁ ȋ Ȍ ǫ Ǧ͙ ȋȌ͙ Ǧ ͚͚͘͘ Ǧ Ǧ͚ ͙ Ǧ͜ Dz Dz Dz ͚͘ȋ͘Ǥ͚͘͘ Ȍ Ǧ͛ ͚ ͠͠ȋ͘Ǥ͚͜͜ Ȍ Ǧ͙ǡǦ͙ǡǦ͚ Dz Dz Dz ͙͘͘ Ǧ͜ ͚ Ǧ Dz Dz Dz ͘Ǥ͙͛ ǡ ǡ Ǧ͝ ȋȌ͜ Ǧ͙ǡǦ͙ǡǦ͛ Dz Dz Dz Ǧ͞ Ǧ͙ Dz Dz ͟ǡ Ǧ͟ ͙ Ǧ Dz ͚͙͛͘ ͙͞ǡ Ǧ͠ ǦǦ Dz ͚͙͘͡ Ǥ ȋ ǡȌ Ǥ ǯ Ǧ͙ ͙͘͟Ǥ͚͛Ǥ ͡Ȁ͙͡Ȁ͚͙͘͞ Ǥ ȋ ǣ ǡ Ȍ Ǥ ǫ Ǥ Ǧ͙ ǡ Ǥ ͙͜ ǡ Ǥ ǣ͝Ȁ͚͞Ȁ͚͚͘͘ȁǣ ͗ǣ͘͘͘͘͜͡͞ǡǣ ȋ Ȍǣ͝Ȁ͙͙Ȁ͚͙͘͠ Ǥ ǡ ǡ ǡ Ǥ ǯ ȋǡ ǡ ǥȌ Ǥ ǫ Ȃ Ǧ͙ ͙͘͜͜͜ ͚͜ ͚͚͙͘ ͙͚͙͙͘͘͠͠ǡ͚͡͞ Ȃ Ǧ͚ ͙͆͜ǡ͚͜͟ ͙͙͛͛͘͘͘͘ ͚͚͙͘ ͘Ǥ͙͚͙͘͘͘͠Ǥ͘͠ Ȃ Ǧ͛ ͆͠͞ǡ͘͘͘ ͙͙͛͛͘͘͘͘ ͚͚͙͘ Ȃ Ǧ͜ Ǧ ͙ ͚͚͙͘ Ǧ͝ ȋ Ȍ Dz Ǥ Ǧ͞ Dz Ǥ ȋǣǦȂ ǢȂǯȌ Ǧǡǡ Ǥ ȋǤǤȀ ȀȀǤȌ Ǧ͙ ȋǤǤȌ ̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸ ͛͘Ǥ Ǧ Ǥ Ǧ͚ Ǧǡ ǡ Dz ǡǡǤ ̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸ ͛͘ Ǥ Ǧ Ǥ Ǧǡ ǡ ͟ Ǧ͛ Ǧ Dz ǡǡǤ to advertising for opportunity to hold a Public Hearing Open House. -

Antioxidant Effects of Schisandra Chinensis Fruits and Their Active Constituents
antioxidants Review Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents Dalia M. Kopustinskiene 1 and Jurga Bernatoniene 1,2,* 1 Institute of Pharmaceutical Technologies, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania; [email protected] 2 Department of Drug Technology and Social Pharmacy, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania * Correspondence: [email protected] Abstract: Schisandra chinensis Turcz. (Baill.) fruits, their extracts, and bioactive compounds are used in alternative medicine as adaptogens and ergogens protecting against numerous neurological, cardiovascular, gastrointestinal, liver, and skin disorders. S. chinensis fruit extracts and their active compounds are potent antioxidants and mitoprotectors exerting anti-inflammatory, antiviral, anti- cancer, and anti-aging effects. S. chinensis polyphenolic compounds—flavonoids, phenolic acids and the major constituents dibenzocyclooctadiene lignans are responsible for the S. chinensis antioxidant activities. This review will focus on the direct and indirect antioxidant effects of S. chinensis fruit extract and its bioactive compounds in the cells during normal and pathological conditions. Keywords: Schisandra chinensis; lignan; schisandrin B; antioxidant; pro-oxidant; mitochondria Citation: Kopustinskiene, D.M.; 1. Introduction Bernatoniene, J. Antioxidant Effects Schisandra chinensis Turcz. (Baill.) belongs to the Schisandraceae family. The plants of Schisandra chinensis Fruits and are native to northeastern China, Japan, Korea, Manchuria, and the Far East part of Russia. Their Active Constituents. Their purple-red berries are called five-flavor fruits because of the sweet, bitter, pungent, Antioxidants 2021, 10, 620. https:// salty, and sour taste [1–5]. S. -

Study on the Modern Application of Schisandra Chinensis
2017 International Conference on Medical Science and Human Health (MSHH 2017) ISBN: 978-1-60595-472-1 Study on the Modern Application of Schisandra Chinensis Ya-Juan WENa, Xiao-Yan FANG, Ming BAI and Ming-San MIAOb,* Henan University of Chinese Medicine, Zhengzhou, 450006, China [email protected], [email protected] *Corresponding author Keywords: Schisandra Chinensis, Chemical Constituents, Pharmacological Action, Clinical Application. Dietotherapy Application. Abstract. This paper analyzes the chemical, pharmacological and application characteristics of Schisandra chinensis, and provides a way for comprehensive utilization of Schisandra chinensis. To summarize the existing experimental and clinical research of Schisandra chinensis, analysis of the characteristics of Schisandra, comprehensive utilization of Schisandra way. The main chemical constituents of Schisandra chinensis, lignans, polysaccharide, volatile oil, Triterpenes, organic acids, amino acids and inorganic elements. It has the advantages of increasing central nervousness, enhancing immunity, cardiovascular system tension and cardiac contractility, and reducing the pharmacological effects of serum alanine aminotransferase (ALT) activity in patients with viral hepatitis. Schisandra has a high medicinal value, its active ingredients in-depth development of research, in particular, the tumor is expected to find active compounds or innovative drugs, for the development of Schisandra food, health products, also has broad prospects. Introduction Schisandra chinensis is the dried ripe fruit of Schisandra chinensis in the magnolia plant. The main production in the northeast, North China, Hubei, Hunan, Jiangxi, Sichuan and other places [1]. Schisandra has the effect of convergence Guse, tonifying qi and promoting blood circulation, tonifying kidney and heart[2]. Commonly used in the treatment of long coughing virtual asthma, dream slippery, injury and thirst, palpitation and insomnia and other symptoms. -

Bay Star-Vine
Common Name: BAY STAR-VINE Scientific Name: Schisandra glabra (Brickell) Rehder Other Commonly Used Names: climbing-magnolia, magnolia-vine Previously Used Scientific Names: Schisandra coccinea Michaux Family: Schisandraceae (star-vine) Rarity Ranks: G3/S2 State Legal Status: Threatened Federal Legal Status: none Federal Wetland Status: none Description: Woody vine, twining up trees and forming low thickets on the ground; bark is gray and bumpy on older vines. Leaves ¾ - 5 inches (2 - 13 cm) long and ⅜ - 3 inches (1 - 8 cm) wide, oval with tapering leaf bases, pointed tips, and widely spaced teeth along the margins; spicy-smelling when crushed. Leaf stalks up to ⅜ - 2¾ inches (1 - 7 cm) long. Female and male flowers are on the same plant, drooping on delicate stalks 1 - 2 inches (2.5 - 5 cm) long; both female and male flowers with 9 - 12 rounded, red and green tepals (petals + sepals). Female flowers with 6 - 12 pistils, male flowers with stamens embedded in a small, flattened disk. Fruit a round or oval, red berry, up to ⅜ inch (4 - 8 mm) wide and ½ inch (0.5 - 1.5 cm) long, dangling in small, loose bunches. Similar Species: Climbing hydrangea (Decumaria barbara) attaches to trees with many, hairy roots; its leaves are opposite, and its white flowers are in flat-topped clusters. Related Rare Species: None in Georgia. Habitat: Moist, deciduous hardwood forests, often with beech, usually on lower slopes, stream terraces, and floodplains. Life History: Bay starvine reproduces vegetatively – by rooting at the nodes of vines sprawling across the ground – and sexually. It is monoecious – male and female reproductive parts are in different flowers on the same plant. -
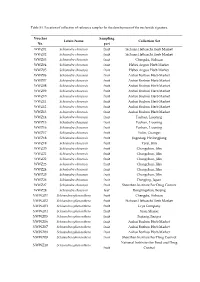
Table S1. Location of Collection of Reference Samples for the Development of the Nucleotide Signature
Table S1. Location of collection of reference samples for the development of the nucleotide signature. Voucher Sampling Latain Name Collection Set No. part WWZ01 Schisandra chinensis fruit Sichuan Hehuachi Herb Market WWZ02 Schisandra chinensis fruit Sichuan Hehuachi Herb Market WWZ03 Schisandra chinensis fruit Chengdu, Sichuan WWZ04 Schisandra chinensis fruit Hebei Anguo Herb Market WWZ05 Schisandra chinensis fruit Hebei Anguo Herb Market WWZ06 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ07 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ08 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ09 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ10 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ11 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ12 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ13 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ14 Schisandra chinensis fruit Fushun, Liaoning WWZ15 Schisandra chinensis fruit Fushun, Liaoning WWZ16 Schisandra chinensis fruit Fushun, Liaoning WWZ17 Schisandra chinensis fruit Yulin, Guangxi WWZ18 Schisandra chinensis fruit Jiagedaqi, Heilongjiang WWZ19 Schisandra chinensis fruit Yanji, Jilin WWZ20 Schisandra chinensis fruit Changchun, Jilin WWZ21 Schisandra chinensis fruit Changchun, Jilin WWZ22 Schisandra chinensis fruit Changchun, Jilin WWZ23 Schisandra chinensis fruit Changchun, Jilin WWZ24 Schisandra chinensis fruit Changchun, Jilin WWZ25 Schisandra chinensis fruit Changchun, Jilin WWZ26 Schisandra chinensis fruit Dongjing, Japan WWZ27 -

Schisandra Chinensis Sam Schmerler
Plainly Unique: Schisandra chinensis Sam Schmerler he plants of the Arnold Arboretum dis- into elongated fruits with numerous bright red, play incredible floral diversity. Magnolia berrylike fruitlets. Winter will reveal exfoliating Tmacrophylla’s huge waxy blooms open bark resembling that of climbing hydrangea. twice, partly closing in between for an over- Evolutionary biologists (including Arboretum night sex change. Helwingia japonica sprouts director Ned Friedman) have discovered that tiny green umbels in the center of otherwise Schisandra and the other Austrobaileyales can unremarkable leaves. Davidia involucrata for- offer insight into many key events in the his- goes petals entirely, but shelters its reproductive tory of flowering plants. Aspects of Schisandra’s organs with massive white bracts. Even wild vascular system may represent an early step in Viola sororia, flagging down bees with its iconic the development of vessels, the structures that violets, surreptitiously sends out discrete, self- allow most flowering plants to rapidly trans- pollinating flowers underground. port water and ecologically dominate hot and With all this bizarre and beautiful reproduc- dry habitats. Schisandra also retains a relatively tion going on, most of us overlook the most simple anatomy during its haploid stage, with evolutionarily distinctive flowering plant in only four nuclei and one developmental module the collection: Schisandra chinensis. An unas- in each female gametophyte (almost all flower- ing plants have eight nuclei and two modules). suming woody vine, it represents a unique and The endosperm of Schisandra seeds conse- ancient lineage that parted ways with most other quently contains only one complement of genes flowering plants at least as far back as the early from each of its parents, while most flowering Cretaceous, before even “living fossils” like plants acquire an additional copy of their moms’ Magnolia. -

NPN Winter 2014.Spub
NATIVE PLANT NEWS the newsletter of the North Carolina Native Plant Society VOLUME 12, ISSUE 1 ISSN: 2151-2159 WINTER 2014 NCNPS Spring Trip 2014: May 16–18: Green Swamp & Lake BE PREPARED for an amazing Spring Trip in 2014, when we will visit some of North Carolina’s most fascinating habitats. Friday afternoon early arrivals will do some highway botanizing along Highways 130 and 17. The Friday night program will be at Lake Waccamaw State Park (1866 State Park Drive, Lake Waccamaw NC 28450), where Park Superintendent Toby Hall will introduce us to the soils and plants of Lake Waccamaw. Saturday David McAdoo, Mark Rose, Angie Carl, and Robert Thornhill will lead us exploring the Green Swamp, which The Nature Conservancy has called “a place unlike any other”. Among the plants we’re likely to see are Venus Fly-trap (Dionaea muscipula), three species of (each) pitcherplants, sundews, and Calopogon orchids; bladderworts, Goldencrest (Lophiola aurea), two species of Cleistes orchid, and much more. It will be a day to remember! The Saturday evening dinner and native plant auction will be at the NC Museum of Forestry (415 S. Madison Street, Whiteville NC 28472) in Whiteville. So start potting up those plants soon! Sunday morning we will visit Lake Waccamaw (1866 State Park Drive, Lake Waccamaw NC 28450), where the park ranger will give us a presentation and then we’ll botanize around the lake. We will wrap up by 1:00 p.m. We’ll be staying at the EconoLodge Hotel in Whiteville, at 503 J. L. Powell Blvd. -

SWAP 2015 Report
STATE WILDLIFE ACTION PLAN September 2015 GEORGIA DEPARTMENT OF NATURAL RESOURCES WILDLIFE RESOURCES DIVISION Georgia State Wildlife Action Plan 2015 Recommended reference: Georgia Department of Natural Resources. 2015. Georgia State Wildlife Action Plan. Social Circle, GA: Georgia Department of Natural Resources. Recommended reference for appendices: Author, A.A., & Author, B.B. Year. Title of Appendix. In Georgia State Wildlife Action Plan (pages of appendix). Social Circle, GA: Georgia Department of Natural Resources. Cover photo credit & description: Photo by Shan Cammack, Georgia Department of Natural Resources Interagency Burn Team in Action! Growing season burn on May 7, 2015 at The Nature Conservancy’s Broxton Rocks Preserve. Zach Wood of The Orianne Society conducting ignition. i Table&of&Contents& Acknowledgements ............................................................................................................ iv! Executive Summary ............................................................................................................ x! I. Introduction and Purpose ................................................................................................. 1! A Plan to Protect Georgia’s Biological Diversity ....................................................... 1! Essential Elements of a State Wildlife Action Plan .................................................... 2! Species of Greatest Conservation Need ...................................................................... 3! Scales of Biological Diversity -

Dynamic Evolution and Phylogenomic Analysis of the Chloroplast Genome
www.nature.com/scientificreports OPEN Dynamic evolution and phylogenomic analysis of the chloroplast genome in Received: 19 March 2018 Accepted: 31 May 2018 Schisandraceae Published: xx xx xxxx Bin Li1,2,3 & Yongqi Zheng1,2,3 Chloroplast genomes of plants are highly conserved in both gene order and gene content, are maternally inherited, and have a lower rate of evolution. Chloroplast genomes are considered to be good models for testing lineage-specifc molecular evolution. In this study, we use Schisandraceae as an example to generate insights into the overall evolutionary dynamics in chloroplast genomes and to establish the phylogenetic relationship of Schisandraceae based on chloroplast genome data using phylogenomic analysis. By comparing three Schisandraceae chloroplast genomes, we demonstrate that the gene order, gene content, and length of chloroplast genomes in Schisandraceae are highly conserved but experience dynamic evolution among species. The number of repeat variations were detected, and the Schisandraceae chloroplast genome was revealed as unusual in having a 10 kb contraction of the IR due to the genome size variations compared with other angiosperms. Phylogenomic analysis based on 82 protein-coding genes from 66 plant taxa clearly elucidated that Schisandraceae is a sister to a clade that includes magnoliids, monocots, and eudicots within angiosperms. As to genus relationships within Schisandraceae, Kadsura and Schisandra formed a monophyletic clade which was sister to Illicium. Chloroplasts are the photosynthetic organelle that provides energy for plants. Te chloroplast has its own genome. In angiosperms, most chloroplast genomes are composed of circular DNA molecules ranging from 120 to 160 kb in length and have a quadripartite organization consisting of two copies of inverted repeats (IRs) of approximately 20–28 kb in size, which divide the rest of chloroplast genome into an 80–90 kb large single copy (LSC) region and a 16–27 kb small single copy (SSC) region. -

Current Knowledge of Schisandra Chinensis (Turcz.) Baill
Phytochem Rev DOI 10.1007/s11101-016-9470-4 Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies Agnieszka Szopa . Radosław Ekiert . Halina Ekiert Received: 11 December 2015 / Accepted: 6 May 2016 © The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Schisandra chinensis Turcz. (Baill.) is a Keywords Schizandra · plant species whose fruits have been well known in Far Dibenzocyclooctadiene lignans · Schisandrin · Eastern medicine for a long time. However, schisandra Gomisin A · Triterpenoids · In vitro cultures seems to be a plant still underestimated in contempo- rary therapy still in the countries of East Asia. The article presents latest available information on the Introduction chemical composition of this plant species. Special attention is given to dibenzo cyclooctadiene lignans. In Schisandra chinensis (Turcz.) Baill.—Chinese mag- addition, recent studies of the biological activity of nolia vine; schisandra (eng.), schizandre de Chine dibenzocyclooctadiene lignans and schisandra fruit (fr.), chinesische Beerentraube, chinesisches Spalt- extracts are recapitulated. The paper gives a short ko¨lbchen (germ.), Лимoнник китaйcкий (rus.), resume of their beneficial effects in biological systems wuweizi, 五味子 (chin.), gomishi, ゴミシ (jap.), 오 in vitro, in animals, and in humans, thus underlining 미자, omija (kor.) is a plant species well-known in their medicinal potential. The cosmetic properties are Traditional Chinese Medicine (TCM) and also in depicted, too. The analytical methods used for assaying modern Chinese medicine. The first description of S. schisandra lignans in the scientific studies and also in chinensis species can be found in a 1596 work on industry are also presented. -

Fall 2005 1 the Lady-Slipper
The Lady-Slipper, 20:3 / Fall 2005 1 The Lady-Slipper Kentucky Native Plant Society Number 20:3 Fall 2005 A Message from the President: NOMINATIONS Hope everyone had a great summer. I spent several weeks roaming the wilds of Michigan and SOUGHT FOR 2006 Minnesota (work projects). Experienced some great plants and natural communities but it’s KENTUCKY always great to come back to Kentucky. WILDFLOWER OF THE Our native plant certification program at Northern Kentucky University is still going great. This YEAR semester, John Thieret is teaching a course on grasses and the students are apparently loving it. For those of you that do not know John, he is one Past recipients: of the country’s leading botanists. He is Professor 1997 Butterfly Milkweed (Asclepias tuberosa) Emeritus at NKU but still does some teaching from 1998 Cardinal Flower (Lobelia cardinalis) time to time. He is also one of the country’s 1999 Purple Coneflower (Echinacea purpurea) leading botanical editors. 2000 Wild Columbine (Aquilegia canadensis) 2001Wild Bergamot (Monarda fistulosa) Later this semester, Deborah White will be teaching 2002 Great Blue Lobelia (Lobelia siphilitica) an elective course on rare plant conservation. 2003 Spiked Blazing Star (Liatris spicata) From what I understand the class is filling up 2004 Joe-Pye Weed (Eupatorium maculatum) quickly. 2005 Showy Goldenrod (Solidago speciosa) Our Fall meeting was a great success with about 45 in attendence. All enjoyed the presentation by Tom Barnes. The Fall colors have given way to 2006 choices: winter buds and piles of leaves. Earlier this fall, Smooth Aster (Symphyotrichum laeve) Lela and I traveled through Asheville NC on our Aromatic Aster (Symphyotrichum oblongifolium) way to Columbia SC and enjoyed a nice slash of Foxglove Beardstongue (Penstemon digitalis) Fall color along the way. -

Georgia's Natural Communities and Associated Rare Plant and Animal Species: Thumbnail Accounts
Georgia's Natural Communities and Associated Rare Plant and Animal Species: Thumbnail Accounts Written by Linda Chafin and based on "Guide to the Natural Communities of Georgia," by Leslie Edwards, Jon Ambrose, and Katherine Kirkman, 2013, University of Georgia Press. Version of 2011 Georgia Nongame Conservation Section Wildlife Resources Division Georgia Department of Natural Resources CONTENTS BLUE RIDGE ECOREGION Upland Forests of the Blue Ridge Blue Ridge northern hardwood and boulderfield forests Blue Ridge montane oak forests Blue Ridge cove forests–fertile variant Blue Ridge cove forests–acidic variant Blue Ridge low to mid-elevation oak forests Blue Ridge pine-oak woodlands Blue Ridge ultramafic barrens and woodlands Glades, Barrens, and Rock Outcrops of the Blue Ridge Ecoregion Blue Ridge rocky summits Blue Ridge cliffs Blue Ridge mafic domes, glades, and barrens Wetlands of the Blue Ridge Ecoregion Blue Ridge mountain bogs Blue Ridge seepage wetlands Blue Ridge spray cliffs Blue Ridge floodplains and bottomlands Aquatic Environments of the Blue Ridge Ecoregion Blue Ridge springs, spring runs, and seeps Blue Ridge small streams Blue Ridge medium to large rivers CUMBERLAND PLATEAU AND RIDGE & VALLEY ECOREGIONS Upland Forests of the Cumberland Plateau and Ridge & Valley Ecoregions Cumberland Plateau and Ridge & Valley mesic forests Cumberland Plateau and Ridge & Valley dry calcareous forests Cumberland Plateau and Ridge & Valley dry oak - pine - hickory forests Cumberland Plateau and Ridge & Valley pine - oak woodlands and forests