Comparative Phytochemical Analysis of Chinese and Bay Starvine (Schisandra Spp.): Potential for Development As a New Dietary Supplement Ingredient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chattahoochee River Pedestrian Bridge: Environmental Assessment
Project No: NA Fulton County P.I. Number 0009640 State Route (SR) 9 at Chattahoochee River in Roswell - Enhancements ENVIRONMENTAL ASSESSMENT U.S. DEPARTMENT OF TRANSPORTATION FEDERAL HIGHWAY ADMINISTRATION AND GEORGIA DEPARTMENT OF TRANSPORTATION SUBMITTED PURSUANT TO 42 USC 4321 et. seq. And 49 USC 303 (for 4(f), if applicable) May 20, 2020 NEPA DATE Eric Duff DATEDAT State Environmental Administrator APPROVAL OF ENVIRONMENTAL ASSESSMENT AND ADVANCEMENT TO AVAILABILITY/PUBLIC HEARING PHASE Digitally signed by JENNIFER L JENNIFER L GIERSCH GIERSCH Date: 2020.06.24 11:59:12 -04'00' DATE FOR: MOISES MARRERO DIVISION ADMINISTRATOR FEDERAL HIGHWAY ADMINISTRATION ǣ͝Ȁ͚͞Ȁ͚͚͘͘ȁǣ ͗ǣ͘͘͘͘͜͡͞ǡǣ ȋ Ȍǣ͝Ȁ͙͙Ȁ͚͙͘͠ 7KHHQJLQHHURIUHFRUG (25 DVVHUWVWKDW ȀǣǤǤ͝Ȁ͚͟Ȁ͚͚͘͘ ǣǤǤ͝Ȁ͚͟Ȁ͚͚͘͘ SODQVLQFRUSRUDWHRUZLOOLQFRUSRUDWHFRPPLWPHQWV 7KH*'27SURMHFWPDQDJHU 30 DVVHUWVWKDW ǣ Ǥ Ǥ͝Ȁ͚͟Ȁ͚͚͘͘ ǣ͝Ȁ͚͞Ȁ͚͚͘͘ WKHVHFRPPLWPHQWVDUHIHDVLEOH LIDSSOLFDEOH ǡ *'2730(ND2NRQPNSDHWRB 6LJQDWXUH'DWH(NDNSDHWR Ǥ (25BBB BBB ǣ͝Ȁ͚͟Ȁ͚͚͘͘ 6LJQDWXUH'DWH Ǥ Ȁ ȋ Ȍ ǫ Ǧ͙ ȋȌ͙ Ǧ ͚͚͘͘ Ǧ Ǧ͚ ͙ Ǧ͜ Dz Dz Dz ͚͘ȋ͘Ǥ͚͘͘ Ȍ Ǧ͛ ͚ ͠͠ȋ͘Ǥ͚͜͜ Ȍ Ǧ͙ǡǦ͙ǡǦ͚ Dz Dz Dz ͙͘͘ Ǧ͜ ͚ Ǧ Dz Dz Dz ͘Ǥ͙͛ ǡ ǡ Ǧ͝ ȋȌ͜ Ǧ͙ǡǦ͙ǡǦ͛ Dz Dz Dz Ǧ͞ Ǧ͙ Dz Dz ͟ǡ Ǧ͟ ͙ Ǧ Dz ͚͙͛͘ ͙͞ǡ Ǧ͠ ǦǦ Dz ͚͙͘͡ Ǥ ȋ ǡȌ Ǥ ǯ Ǧ͙ ͙͘͟Ǥ͚͛Ǥ ͡Ȁ͙͡Ȁ͚͙͘͞ Ǥ ȋ ǣ ǡ Ȍ Ǥ ǫ Ǥ Ǧ͙ ǡ Ǥ ͙͜ ǡ Ǥ ǣ͝Ȁ͚͞Ȁ͚͚͘͘ȁǣ ͗ǣ͘͘͘͘͜͡͞ǡǣ ȋ Ȍǣ͝Ȁ͙͙Ȁ͚͙͘͠ Ǥ ǡ ǡ ǡ Ǥ ǯ ȋǡ ǡ ǥȌ Ǥ ǫ Ȃ Ǧ͙ ͙͘͜͜͜ ͚͜ ͚͚͙͘ ͙͚͙͙͘͘͠͠ǡ͚͡͞ Ȃ Ǧ͚ ͙͆͜ǡ͚͜͟ ͙͙͛͛͘͘͘͘ ͚͚͙͘ ͘Ǥ͙͚͙͘͘͘͠Ǥ͘͠ Ȃ Ǧ͛ ͆͠͞ǡ͘͘͘ ͙͙͛͛͘͘͘͘ ͚͚͙͘ Ȃ Ǧ͜ Ǧ ͙ ͚͚͙͘ Ǧ͝ ȋ Ȍ Dz Ǥ Ǧ͞ Dz Ǥ ȋǣǦȂ ǢȂǯȌ Ǧǡǡ Ǥ ȋǤǤȀ ȀȀǤȌ Ǧ͙ ȋǤǤȌ ̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸ ͛͘Ǥ Ǧ Ǥ Ǧ͚ Ǧǡ ǡ Dz ǡǡǤ ̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸ ͛͘ Ǥ Ǧ Ǥ Ǧǡ ǡ ͟ Ǧ͛ Ǧ Dz ǡǡǤ to advertising for opportunity to hold a Public Hearing Open House. -

Antioxidant Effects of Schisandra Chinensis Fruits and Their Active Constituents
antioxidants Review Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents Dalia M. Kopustinskiene 1 and Jurga Bernatoniene 1,2,* 1 Institute of Pharmaceutical Technologies, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania; [email protected] 2 Department of Drug Technology and Social Pharmacy, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania * Correspondence: [email protected] Abstract: Schisandra chinensis Turcz. (Baill.) fruits, their extracts, and bioactive compounds are used in alternative medicine as adaptogens and ergogens protecting against numerous neurological, cardiovascular, gastrointestinal, liver, and skin disorders. S. chinensis fruit extracts and their active compounds are potent antioxidants and mitoprotectors exerting anti-inflammatory, antiviral, anti- cancer, and anti-aging effects. S. chinensis polyphenolic compounds—flavonoids, phenolic acids and the major constituents dibenzocyclooctadiene lignans are responsible for the S. chinensis antioxidant activities. This review will focus on the direct and indirect antioxidant effects of S. chinensis fruit extract and its bioactive compounds in the cells during normal and pathological conditions. Keywords: Schisandra chinensis; lignan; schisandrin B; antioxidant; pro-oxidant; mitochondria Citation: Kopustinskiene, D.M.; 1. Introduction Bernatoniene, J. Antioxidant Effects Schisandra chinensis Turcz. (Baill.) belongs to the Schisandraceae family. The plants of Schisandra chinensis Fruits and are native to northeastern China, Japan, Korea, Manchuria, and the Far East part of Russia. Their Active Constituents. Their purple-red berries are called five-flavor fruits because of the sweet, bitter, pungent, Antioxidants 2021, 10, 620. https:// salty, and sour taste [1–5]. S. -

Study on the Modern Application of Schisandra Chinensis
2017 International Conference on Medical Science and Human Health (MSHH 2017) ISBN: 978-1-60595-472-1 Study on the Modern Application of Schisandra Chinensis Ya-Juan WENa, Xiao-Yan FANG, Ming BAI and Ming-San MIAOb,* Henan University of Chinese Medicine, Zhengzhou, 450006, China [email protected], [email protected] *Corresponding author Keywords: Schisandra Chinensis, Chemical Constituents, Pharmacological Action, Clinical Application. Dietotherapy Application. Abstract. This paper analyzes the chemical, pharmacological and application characteristics of Schisandra chinensis, and provides a way for comprehensive utilization of Schisandra chinensis. To summarize the existing experimental and clinical research of Schisandra chinensis, analysis of the characteristics of Schisandra, comprehensive utilization of Schisandra way. The main chemical constituents of Schisandra chinensis, lignans, polysaccharide, volatile oil, Triterpenes, organic acids, amino acids and inorganic elements. It has the advantages of increasing central nervousness, enhancing immunity, cardiovascular system tension and cardiac contractility, and reducing the pharmacological effects of serum alanine aminotransferase (ALT) activity in patients with viral hepatitis. Schisandra has a high medicinal value, its active ingredients in-depth development of research, in particular, the tumor is expected to find active compounds or innovative drugs, for the development of Schisandra food, health products, also has broad prospects. Introduction Schisandra chinensis is the dried ripe fruit of Schisandra chinensis in the magnolia plant. The main production in the northeast, North China, Hubei, Hunan, Jiangxi, Sichuan and other places [1]. Schisandra has the effect of convergence Guse, tonifying qi and promoting blood circulation, tonifying kidney and heart[2]. Commonly used in the treatment of long coughing virtual asthma, dream slippery, injury and thirst, palpitation and insomnia and other symptoms. -

Vascular Plant Families of the United States Grouped by Diagnostic Features
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 12-6-2019 Vascular Plant Families of the United States Grouped by Diagnostic Features James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Vascular Plant Families of the United States Grouped by Diagnostic Features" (2019). Botanical Studies. 96. https://digitalcommons.humboldt.edu/botany_jps/96 This Flora of the United States and North America is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. FLOWERING PLANT FAMILIES OF THE UNITED STATES GROUPED BY DIAGNOSTIC FEATURES James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Second edition — 6 December 2019 The focus is on families of plants found in the conterminous United States, including ornamentals. The listing of a family is not meant to imply that every species has that feature. I am using a fewfamily names, such as Liliaceae, Plantaginaceae, and Scrophulariaceae, in the traditional sense, because their limits remain unsettled. Parasitic on branches Dioscoreaceae -

Bay Star-Vine
Common Name: BAY STAR-VINE Scientific Name: Schisandra glabra (Brickell) Rehder Other Commonly Used Names: climbing-magnolia, magnolia-vine Previously Used Scientific Names: Schisandra coccinea Michaux Family: Schisandraceae (star-vine) Rarity Ranks: G3/S2 State Legal Status: Threatened Federal Legal Status: none Federal Wetland Status: none Description: Woody vine, twining up trees and forming low thickets on the ground; bark is gray and bumpy on older vines. Leaves ¾ - 5 inches (2 - 13 cm) long and ⅜ - 3 inches (1 - 8 cm) wide, oval with tapering leaf bases, pointed tips, and widely spaced teeth along the margins; spicy-smelling when crushed. Leaf stalks up to ⅜ - 2¾ inches (1 - 7 cm) long. Female and male flowers are on the same plant, drooping on delicate stalks 1 - 2 inches (2.5 - 5 cm) long; both female and male flowers with 9 - 12 rounded, red and green tepals (petals + sepals). Female flowers with 6 - 12 pistils, male flowers with stamens embedded in a small, flattened disk. Fruit a round or oval, red berry, up to ⅜ inch (4 - 8 mm) wide and ½ inch (0.5 - 1.5 cm) long, dangling in small, loose bunches. Similar Species: Climbing hydrangea (Decumaria barbara) attaches to trees with many, hairy roots; its leaves are opposite, and its white flowers are in flat-topped clusters. Related Rare Species: None in Georgia. Habitat: Moist, deciduous hardwood forests, often with beech, usually on lower slopes, stream terraces, and floodplains. Life History: Bay starvine reproduces vegetatively – by rooting at the nodes of vines sprawling across the ground – and sexually. It is monoecious – male and female reproductive parts are in different flowers on the same plant. -

Read Book Schisandra Chinensis: an Herb of North Eastern China Origin
SCHISANDRA CHINENSIS: AN HERB OF NORTH EASTERN CHINA ORIGIN PDF, EPUB, EBOOK Kam Ming Ko | 252 pages | 28 Feb 2015 | World Scientific Publishing Co Pte Ltd | 9789814651226 | English | Singapore, Singapore Schisandra Chinensis: An Herb Of North Eastern China Origin PDF Book Due to its ability to positively affect the immune system and fight inflammation , schisandra seems to help stall the development of atherosclerosis hardening of the arteries , balances blood sugar, prevents diabetes and bring the body into an optimal acid-base balance. Safety: High, but may cause over-stimulation if overused. The average increase in the mean concentration of Tac in the blood was percent for the group receiving higher doses of SchE and percent for the group receiving lower a dose. Repeated expansions and fragmentations linked to Pleistocene climate changes shaped the genetic structure of a woody climber, Actinidia arguta Actinidiaceae. Structure-activity relationships of lignans from Schisandra chinensis as platelet activating antagonists. All rights reserved. Schisandra berry demonstrates significant adaptogenic activity. To assure optimal quality, they directly purchase from China berries that have been graded as premium quality only. But some berries are deep refrigerated, and eventually used to make health juices, primarily for the Korean market. Schisandra supports the immune system and protects against microbes. Schisandra chinensis can also function as a convalescent tonic herb when the kidney system is involved. Evolution 38, — Antao, T. Share: facebook twitter pinterest. We recommend that you consult with a qualified healthcare practitioner before using herbal products, particularly if you are pregnant, nursing, or on any medications. When it comes to cancer prevention, active lignans have been isolated from schisandra especially one called schisandrin A that have chemo-protective abilities. -

Characterization of Two PEBP Genes, Srft and Srmft, in Thermogenic
www.nature.com/scientificreports OPEN Characterization of two PEBP genes, SrFT and SrMFT, in thermogenic skunk cabbage Received: 09 March 2016 Accepted: 20 June 2016 (Symplocarpus renifolius) Published: 08 July 2016 Yasuko Ito-Inaba1, Hiromi Masuko-Suzuki2, Haruhiko Maekawa1,3, Masao Watanabe2 & Takehito Inaba3 Floral thermogenesis has been found in dozens of primitive seed plants and the reproductive organs in these plants produce heat during anthesis. Thus, characterization of the molecular mechanisms underlying flowering is required to fully understand the role of thermogenesis, but this aspect of thermogenic plant development is largely unknown. In this study, extensive database searches and cloning experiments suggest that thermogenic skunk cabbage (Symplocarpus renifolius), which is a member of the family Araceae, possesses two genes encoding phosphatidyl ethanolamine-binding proteins (PEBP), FLOWERING LOCUS T (SrFT) and MOTHER OF FT AND TFL1 (SrMFT). Functional analyses of SrFT and SrMFT in Arabidopsis indicate that SrFT promotes flowering, whereasSrMFT does not. In S. renifolius, the stage- and tissue-specific expression ofSrFT was more evident than that of SrMFT. SrFT was highly expressed in flowers and leaves and was mainly localized in fibrovascular tissues. In addition, microarray analysis revealed that, within floral tissues,SrFT was co-regulated with the genes associated with cellular respiration and mitochondrial function, including ALTERNATIVE OXIDASE gene proposed to play a major role in floral thermogenesis. Taken together, these data suggest that, among the PEBP genes, SrFT plays a role in flowering and floral development in the thermogenic skunk cabbage. Floral thermogenesis has been found in dozens of flowering plants including basal angiosperms (Nymphaeaceae and Schisandraceae)1–4, magnoliids (Magnoliaceae)5–7, monocots (Araceae)8–10, and eudicots (Nelumbonaceae and Rafflesiaceae)10,11. -

Aniseed, Were Used As Traditional Medicine in China As Early As in the 5Th Century
Asian J. Med. Biol. Res. 2019, 5 (3), 162-179; doi: 10.3329/ajmbr.v5i3.43584 Asian Journal of Medical and Biological Research ISSN 2411-4472 (Print) 2412-5571 (Online) www.ebupress.com/journal/ajmbr Review Chinese star anise and anise, magic herbs in traditional Chinese medicine and modern pharmaceutical science Mohamad Hesam Shahrajabian1,2, Wenli Sun1,2 and Qi Cheng1,2* 1Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China 2Nitrogen Fixation Laboratory, Qi Institute, Building C4, No.555 Chuangye Road, Jiaxing 314000, Zhejiang, China *Corresponding author: Qi Cheng, Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China. E-mail: [email protected] Received: 05 August 2019/Accepted: 08 September 2019/ Published: 30 September 2019 Abstract: Star anise (Illicium verum Hook. f.) is an important herb in traditional Chinese medicine as well as traditional Asian medicine. The fruit is aromatic and has a strong, pungent and mildly sweet taste. Star anise is one of the many species that contain bioactive compounds as well as a number of phenolic and flavonoid compounds, having antioxidant, preservative and antimicrobial properties. All relevant papers in the English language from researchers of different countries were collected. The keywords of Chinese star anise, anise, traditional Chinese medicine and modern pharmaceutical science were searched in Google Scholar, Scopus, Research Gate and PubMed. Its seeds are good source of minerals like calcium, iron, copper, -
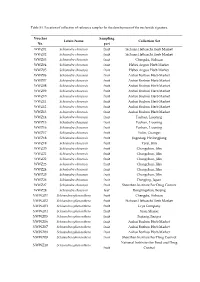
Table S1. Location of Collection of Reference Samples for the Development of the Nucleotide Signature
Table S1. Location of collection of reference samples for the development of the nucleotide signature. Voucher Sampling Latain Name Collection Set No. part WWZ01 Schisandra chinensis fruit Sichuan Hehuachi Herb Market WWZ02 Schisandra chinensis fruit Sichuan Hehuachi Herb Market WWZ03 Schisandra chinensis fruit Chengdu, Sichuan WWZ04 Schisandra chinensis fruit Hebei Anguo Herb Market WWZ05 Schisandra chinensis fruit Hebei Anguo Herb Market WWZ06 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ07 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ08 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ09 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ10 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ11 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ12 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ13 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ14 Schisandra chinensis fruit Fushun, Liaoning WWZ15 Schisandra chinensis fruit Fushun, Liaoning WWZ16 Schisandra chinensis fruit Fushun, Liaoning WWZ17 Schisandra chinensis fruit Yulin, Guangxi WWZ18 Schisandra chinensis fruit Jiagedaqi, Heilongjiang WWZ19 Schisandra chinensis fruit Yanji, Jilin WWZ20 Schisandra chinensis fruit Changchun, Jilin WWZ21 Schisandra chinensis fruit Changchun, Jilin WWZ22 Schisandra chinensis fruit Changchun, Jilin WWZ23 Schisandra chinensis fruit Changchun, Jilin WWZ24 Schisandra chinensis fruit Changchun, Jilin WWZ25 Schisandra chinensis fruit Changchun, Jilin WWZ26 Schisandra chinensis fruit Dongjing, Japan WWZ27 -

Characterization of the Omija (Schisandra Chinensis) Extract and Its Effects on the Bovine Sperm Vitality and Oxidative Profile During in Vitro Storage
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2020, Article ID 7123780, 15 pages https://doi.org/10.1155/2020/7123780 Research Article Characterization of the Omija (Schisandra chinensis) Extract and Its Effects on the Bovine Sperm Vitality and Oxidative Profile during In Vitro Storage Eva Tvrda´ ,1 Jaroslav Michalko,2,3 Ju´ lius A´ rvay,4 Nenad L. Vukovic,5 Eva Ivanisˇova´,6 Michal Dˇ uracˇka,1 Ildiko´ Matusˇı´kova´,7 and Miroslava Kacˇa´niova´ 8,9 1Department of Animal Physiology, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia 2BioFood Center, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia 3Detached Branch of the Institute of Forest Ecology-Arbore´tum Mlynˇany, Slovak Academy of Sciences, Vieska Nad Zˇitavou 178, 951 52 Slepˇcany, Slovakia 4Department of Chemistry, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia 5Department of Chemistry, Faculty of Science, University of Kragujevac, 34000 Kragujevac, Serbia 6Department of Technology and Quality of Plant Products, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture, Tr. A. Hlinku 2, 94976 Nitra, Slovakia 7Department of Ecochemistry and Radioecology, Faculty of Natural Sciences, University of SS. Cyril and Methodius in Trnava, Na´m. J. Herdu 2, 917 01 Trnava, Slovakia 8Department of Fruit Sciences, Viticulture and Enology, Faculty of Horticulture and Landscape Engineering, Slovak University of Agriculture, Tr. A. Hlinku 2, 94976 Nitra, Slovakia 9Department of Bioenergy, Food Technology and Microbiology, Institute of Food Technology and Nutrition, University of Rzeszow, Zelwerowicza St. -

Star Anise – Illicium Verum
Did You Know? Star anise – Illicium verum • The eight-pointed seed pod from an evergreen tree native to Southwest China and Vietnam, is the spice known as star anise. This small evergreen tree is in the magnolia family, Schisandraceae. • Star anise has been used in China for flavoring and medicine for over three thousand years. • The seed pods are harvested before ripening (green) and sun-dried, resulting in the rich brown color. • Both the seeds and the pods contain the flavor and are finely ground together. When used in recipes whole, they should be removed before serving. • It is one of the five spices in the blend, Chinese five-spice. • The deep licorice-like aroma has subtle sweet and herbal notes. • The flavor is used in sweet, spicy and savory dishes, including baked goods, chilled desserts, sauces, beverages and even red meats. • The liquors absinthe, Sambuca, and pastis all have infused star anise flavoring. • Though the flavor is similar, it is not related to anise seed. However, both plants have anethole, a compound responsible for the anise flavor in both seeds. • Historical medicinal uses included Chinese herbalists using star anise as a stimulant, an expectorant and to treat indigestion to European healers using it in teas for rheumatism and chewing the seed for indigestion. • Though there is now a synthetic way to manufacture it, star anise contains shikimic acid which is one of the primary components of the influenza-fighting drug Tamiflu. • Research continues on extracts from star anise, including testing antifungals and antimicrobial compounds. • According to Chinese folklore, finding a star anise with more than eight points was considered good luck. -

Schisandra Chinensis Sam Schmerler
Plainly Unique: Schisandra chinensis Sam Schmerler he plants of the Arnold Arboretum dis- into elongated fruits with numerous bright red, play incredible floral diversity. Magnolia berrylike fruitlets. Winter will reveal exfoliating Tmacrophylla’s huge waxy blooms open bark resembling that of climbing hydrangea. twice, partly closing in between for an over- Evolutionary biologists (including Arboretum night sex change. Helwingia japonica sprouts director Ned Friedman) have discovered that tiny green umbels in the center of otherwise Schisandra and the other Austrobaileyales can unremarkable leaves. Davidia involucrata for- offer insight into many key events in the his- goes petals entirely, but shelters its reproductive tory of flowering plants. Aspects of Schisandra’s organs with massive white bracts. Even wild vascular system may represent an early step in Viola sororia, flagging down bees with its iconic the development of vessels, the structures that violets, surreptitiously sends out discrete, self- allow most flowering plants to rapidly trans- pollinating flowers underground. port water and ecologically dominate hot and With all this bizarre and beautiful reproduc- dry habitats. Schisandra also retains a relatively tion going on, most of us overlook the most simple anatomy during its haploid stage, with evolutionarily distinctive flowering plant in only four nuclei and one developmental module the collection: Schisandra chinensis. An unas- in each female gametophyte (almost all flower- ing plants have eight nuclei and two modules). suming woody vine, it represents a unique and The endosperm of Schisandra seeds conse- ancient lineage that parted ways with most other quently contains only one complement of genes flowering plants at least as far back as the early from each of its parents, while most flowering Cretaceous, before even “living fossils” like plants acquire an additional copy of their moms’ Magnolia.