Current Knowledge of Schisandra Chinensis (Turcz.) Baill
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chattahoochee River Pedestrian Bridge: Environmental Assessment
Project No: NA Fulton County P.I. Number 0009640 State Route (SR) 9 at Chattahoochee River in Roswell - Enhancements ENVIRONMENTAL ASSESSMENT U.S. DEPARTMENT OF TRANSPORTATION FEDERAL HIGHWAY ADMINISTRATION AND GEORGIA DEPARTMENT OF TRANSPORTATION SUBMITTED PURSUANT TO 42 USC 4321 et. seq. And 49 USC 303 (for 4(f), if applicable) May 20, 2020 NEPA DATE Eric Duff DATEDAT State Environmental Administrator APPROVAL OF ENVIRONMENTAL ASSESSMENT AND ADVANCEMENT TO AVAILABILITY/PUBLIC HEARING PHASE Digitally signed by JENNIFER L JENNIFER L GIERSCH GIERSCH Date: 2020.06.24 11:59:12 -04'00' DATE FOR: MOISES MARRERO DIVISION ADMINISTRATOR FEDERAL HIGHWAY ADMINISTRATION ǣ͝Ȁ͚͞Ȁ͚͚͘͘ȁǣ ͗ǣ͘͘͘͘͜͡͞ǡǣ ȋ Ȍǣ͝Ȁ͙͙Ȁ͚͙͘͠ 7KHHQJLQHHURIUHFRUG (25 DVVHUWVWKDW ȀǣǤǤ͝Ȁ͚͟Ȁ͚͚͘͘ ǣǤǤ͝Ȁ͚͟Ȁ͚͚͘͘ SODQVLQFRUSRUDWHRUZLOOLQFRUSRUDWHFRPPLWPHQWV 7KH*'27SURMHFWPDQDJHU 30 DVVHUWVWKDW ǣ Ǥ Ǥ͝Ȁ͚͟Ȁ͚͚͘͘ ǣ͝Ȁ͚͞Ȁ͚͚͘͘ WKHVHFRPPLWPHQWVDUHIHDVLEOH LIDSSOLFDEOH ǡ *'2730(ND2NRQPNSDHWRB 6LJQDWXUH'DWH(NDNSDHWR Ǥ (25BBB BBB ǣ͝Ȁ͚͟Ȁ͚͚͘͘ 6LJQDWXUH'DWH Ǥ Ȁ ȋ Ȍ ǫ Ǧ͙ ȋȌ͙ Ǧ ͚͚͘͘ Ǧ Ǧ͚ ͙ Ǧ͜ Dz Dz Dz ͚͘ȋ͘Ǥ͚͘͘ Ȍ Ǧ͛ ͚ ͠͠ȋ͘Ǥ͚͜͜ Ȍ Ǧ͙ǡǦ͙ǡǦ͚ Dz Dz Dz ͙͘͘ Ǧ͜ ͚ Ǧ Dz Dz Dz ͘Ǥ͙͛ ǡ ǡ Ǧ͝ ȋȌ͜ Ǧ͙ǡǦ͙ǡǦ͛ Dz Dz Dz Ǧ͞ Ǧ͙ Dz Dz ͟ǡ Ǧ͟ ͙ Ǧ Dz ͚͙͛͘ ͙͞ǡ Ǧ͠ ǦǦ Dz ͚͙͘͡ Ǥ ȋ ǡȌ Ǥ ǯ Ǧ͙ ͙͘͟Ǥ͚͛Ǥ ͡Ȁ͙͡Ȁ͚͙͘͞ Ǥ ȋ ǣ ǡ Ȍ Ǥ ǫ Ǥ Ǧ͙ ǡ Ǥ ͙͜ ǡ Ǥ ǣ͝Ȁ͚͞Ȁ͚͚͘͘ȁǣ ͗ǣ͘͘͘͘͜͡͞ǡǣ ȋ Ȍǣ͝Ȁ͙͙Ȁ͚͙͘͠ Ǥ ǡ ǡ ǡ Ǥ ǯ ȋǡ ǡ ǥȌ Ǥ ǫ Ȃ Ǧ͙ ͙͘͜͜͜ ͚͜ ͚͚͙͘ ͙͚͙͙͘͘͠͠ǡ͚͡͞ Ȃ Ǧ͚ ͙͆͜ǡ͚͜͟ ͙͙͛͛͘͘͘͘ ͚͚͙͘ ͘Ǥ͙͚͙͘͘͘͠Ǥ͘͠ Ȃ Ǧ͛ ͆͠͞ǡ͘͘͘ ͙͙͛͛͘͘͘͘ ͚͚͙͘ Ȃ Ǧ͜ Ǧ ͙ ͚͚͙͘ Ǧ͝ ȋ Ȍ Dz Ǥ Ǧ͞ Dz Ǥ ȋǣǦȂ ǢȂǯȌ Ǧǡǡ Ǥ ȋǤǤȀ ȀȀǤȌ Ǧ͙ ȋǤǤȌ ̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸ ͛͘Ǥ Ǧ Ǥ Ǧ͚ Ǧǡ ǡ Dz ǡǡǤ ̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸̸ ͛͘ Ǥ Ǧ Ǥ Ǧǡ ǡ ͟ Ǧ͛ Ǧ Dz ǡǡǤ to advertising for opportunity to hold a Public Hearing Open House. -

Antioxidant Effects of Schisandra Chinensis Fruits and Their Active Constituents
antioxidants Review Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents Dalia M. Kopustinskiene 1 and Jurga Bernatoniene 1,2,* 1 Institute of Pharmaceutical Technologies, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania; [email protected] 2 Department of Drug Technology and Social Pharmacy, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania * Correspondence: [email protected] Abstract: Schisandra chinensis Turcz. (Baill.) fruits, their extracts, and bioactive compounds are used in alternative medicine as adaptogens and ergogens protecting against numerous neurological, cardiovascular, gastrointestinal, liver, and skin disorders. S. chinensis fruit extracts and their active compounds are potent antioxidants and mitoprotectors exerting anti-inflammatory, antiviral, anti- cancer, and anti-aging effects. S. chinensis polyphenolic compounds—flavonoids, phenolic acids and the major constituents dibenzocyclooctadiene lignans are responsible for the S. chinensis antioxidant activities. This review will focus on the direct and indirect antioxidant effects of S. chinensis fruit extract and its bioactive compounds in the cells during normal and pathological conditions. Keywords: Schisandra chinensis; lignan; schisandrin B; antioxidant; pro-oxidant; mitochondria Citation: Kopustinskiene, D.M.; 1. Introduction Bernatoniene, J. Antioxidant Effects Schisandra chinensis Turcz. (Baill.) belongs to the Schisandraceae family. The plants of Schisandra chinensis Fruits and are native to northeastern China, Japan, Korea, Manchuria, and the Far East part of Russia. Their Active Constituents. Their purple-red berries are called five-flavor fruits because of the sweet, bitter, pungent, Antioxidants 2021, 10, 620. https:// salty, and sour taste [1–5]. S. -

Study on the Modern Application of Schisandra Chinensis
2017 International Conference on Medical Science and Human Health (MSHH 2017) ISBN: 978-1-60595-472-1 Study on the Modern Application of Schisandra Chinensis Ya-Juan WENa, Xiao-Yan FANG, Ming BAI and Ming-San MIAOb,* Henan University of Chinese Medicine, Zhengzhou, 450006, China [email protected], [email protected] *Corresponding author Keywords: Schisandra Chinensis, Chemical Constituents, Pharmacological Action, Clinical Application. Dietotherapy Application. Abstract. This paper analyzes the chemical, pharmacological and application characteristics of Schisandra chinensis, and provides a way for comprehensive utilization of Schisandra chinensis. To summarize the existing experimental and clinical research of Schisandra chinensis, analysis of the characteristics of Schisandra, comprehensive utilization of Schisandra way. The main chemical constituents of Schisandra chinensis, lignans, polysaccharide, volatile oil, Triterpenes, organic acids, amino acids and inorganic elements. It has the advantages of increasing central nervousness, enhancing immunity, cardiovascular system tension and cardiac contractility, and reducing the pharmacological effects of serum alanine aminotransferase (ALT) activity in patients with viral hepatitis. Schisandra has a high medicinal value, its active ingredients in-depth development of research, in particular, the tumor is expected to find active compounds or innovative drugs, for the development of Schisandra food, health products, also has broad prospects. Introduction Schisandra chinensis is the dried ripe fruit of Schisandra chinensis in the magnolia plant. The main production in the northeast, North China, Hubei, Hunan, Jiangxi, Sichuan and other places [1]. Schisandra has the effect of convergence Guse, tonifying qi and promoting blood circulation, tonifying kidney and heart[2]. Commonly used in the treatment of long coughing virtual asthma, dream slippery, injury and thirst, palpitation and insomnia and other symptoms. -

Bay Star-Vine
Common Name: BAY STAR-VINE Scientific Name: Schisandra glabra (Brickell) Rehder Other Commonly Used Names: climbing-magnolia, magnolia-vine Previously Used Scientific Names: Schisandra coccinea Michaux Family: Schisandraceae (star-vine) Rarity Ranks: G3/S2 State Legal Status: Threatened Federal Legal Status: none Federal Wetland Status: none Description: Woody vine, twining up trees and forming low thickets on the ground; bark is gray and bumpy on older vines. Leaves ¾ - 5 inches (2 - 13 cm) long and ⅜ - 3 inches (1 - 8 cm) wide, oval with tapering leaf bases, pointed tips, and widely spaced teeth along the margins; spicy-smelling when crushed. Leaf stalks up to ⅜ - 2¾ inches (1 - 7 cm) long. Female and male flowers are on the same plant, drooping on delicate stalks 1 - 2 inches (2.5 - 5 cm) long; both female and male flowers with 9 - 12 rounded, red and green tepals (petals + sepals). Female flowers with 6 - 12 pistils, male flowers with stamens embedded in a small, flattened disk. Fruit a round or oval, red berry, up to ⅜ inch (4 - 8 mm) wide and ½ inch (0.5 - 1.5 cm) long, dangling in small, loose bunches. Similar Species: Climbing hydrangea (Decumaria barbara) attaches to trees with many, hairy roots; its leaves are opposite, and its white flowers are in flat-topped clusters. Related Rare Species: None in Georgia. Habitat: Moist, deciduous hardwood forests, often with beech, usually on lower slopes, stream terraces, and floodplains. Life History: Bay starvine reproduces vegetatively – by rooting at the nodes of vines sprawling across the ground – and sexually. It is monoecious – male and female reproductive parts are in different flowers on the same plant. -

Read Book Schisandra Chinensis: an Herb of North Eastern China Origin
SCHISANDRA CHINENSIS: AN HERB OF NORTH EASTERN CHINA ORIGIN PDF, EPUB, EBOOK Kam Ming Ko | 252 pages | 28 Feb 2015 | World Scientific Publishing Co Pte Ltd | 9789814651226 | English | Singapore, Singapore Schisandra Chinensis: An Herb Of North Eastern China Origin PDF Book Due to its ability to positively affect the immune system and fight inflammation , schisandra seems to help stall the development of atherosclerosis hardening of the arteries , balances blood sugar, prevents diabetes and bring the body into an optimal acid-base balance. Safety: High, but may cause over-stimulation if overused. The average increase in the mean concentration of Tac in the blood was percent for the group receiving higher doses of SchE and percent for the group receiving lower a dose. Repeated expansions and fragmentations linked to Pleistocene climate changes shaped the genetic structure of a woody climber, Actinidia arguta Actinidiaceae. Structure-activity relationships of lignans from Schisandra chinensis as platelet activating antagonists. All rights reserved. Schisandra berry demonstrates significant adaptogenic activity. To assure optimal quality, they directly purchase from China berries that have been graded as premium quality only. But some berries are deep refrigerated, and eventually used to make health juices, primarily for the Korean market. Schisandra supports the immune system and protects against microbes. Schisandra chinensis can also function as a convalescent tonic herb when the kidney system is involved. Evolution 38, — Antao, T. Share: facebook twitter pinterest. We recommend that you consult with a qualified healthcare practitioner before using herbal products, particularly if you are pregnant, nursing, or on any medications. When it comes to cancer prevention, active lignans have been isolated from schisandra especially one called schisandrin A that have chemo-protective abilities. -
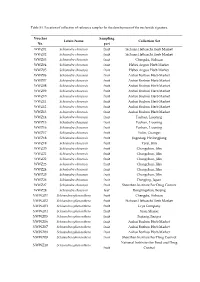
Table S1. Location of Collection of Reference Samples for the Development of the Nucleotide Signature
Table S1. Location of collection of reference samples for the development of the nucleotide signature. Voucher Sampling Latain Name Collection Set No. part WWZ01 Schisandra chinensis fruit Sichuan Hehuachi Herb Market WWZ02 Schisandra chinensis fruit Sichuan Hehuachi Herb Market WWZ03 Schisandra chinensis fruit Chengdu, Sichuan WWZ04 Schisandra chinensis fruit Hebei Anguo Herb Market WWZ05 Schisandra chinensis fruit Hebei Anguo Herb Market WWZ06 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ07 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ08 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ09 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ10 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ11 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ12 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ13 Schisandra chinensis fruit Anhui Bozhou Herb Market WWZ14 Schisandra chinensis fruit Fushun, Liaoning WWZ15 Schisandra chinensis fruit Fushun, Liaoning WWZ16 Schisandra chinensis fruit Fushun, Liaoning WWZ17 Schisandra chinensis fruit Yulin, Guangxi WWZ18 Schisandra chinensis fruit Jiagedaqi, Heilongjiang WWZ19 Schisandra chinensis fruit Yanji, Jilin WWZ20 Schisandra chinensis fruit Changchun, Jilin WWZ21 Schisandra chinensis fruit Changchun, Jilin WWZ22 Schisandra chinensis fruit Changchun, Jilin WWZ23 Schisandra chinensis fruit Changchun, Jilin WWZ24 Schisandra chinensis fruit Changchun, Jilin WWZ25 Schisandra chinensis fruit Changchun, Jilin WWZ26 Schisandra chinensis fruit Dongjing, Japan WWZ27 -

Characterization of the Omija (Schisandra Chinensis) Extract and Its Effects on the Bovine Sperm Vitality and Oxidative Profile During in Vitro Storage
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2020, Article ID 7123780, 15 pages https://doi.org/10.1155/2020/7123780 Research Article Characterization of the Omija (Schisandra chinensis) Extract and Its Effects on the Bovine Sperm Vitality and Oxidative Profile during In Vitro Storage Eva Tvrda´ ,1 Jaroslav Michalko,2,3 Ju´ lius A´ rvay,4 Nenad L. Vukovic,5 Eva Ivanisˇova´,6 Michal Dˇ uracˇka,1 Ildiko´ Matusˇı´kova´,7 and Miroslava Kacˇa´niova´ 8,9 1Department of Animal Physiology, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia 2BioFood Center, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia 3Detached Branch of the Institute of Forest Ecology-Arbore´tum Mlynˇany, Slovak Academy of Sciences, Vieska Nad Zˇitavou 178, 951 52 Slepˇcany, Slovakia 4Department of Chemistry, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture in Nitra, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia 5Department of Chemistry, Faculty of Science, University of Kragujevac, 34000 Kragujevac, Serbia 6Department of Technology and Quality of Plant Products, Faculty of Biotechnology and Food Sciences, Slovak University of Agriculture, Tr. A. Hlinku 2, 94976 Nitra, Slovakia 7Department of Ecochemistry and Radioecology, Faculty of Natural Sciences, University of SS. Cyril and Methodius in Trnava, Na´m. J. Herdu 2, 917 01 Trnava, Slovakia 8Department of Fruit Sciences, Viticulture and Enology, Faculty of Horticulture and Landscape Engineering, Slovak University of Agriculture, Tr. A. Hlinku 2, 94976 Nitra, Slovakia 9Department of Bioenergy, Food Technology and Microbiology, Institute of Food Technology and Nutrition, University of Rzeszow, Zelwerowicza St. -

Schisandra Chinensis Sam Schmerler
Plainly Unique: Schisandra chinensis Sam Schmerler he plants of the Arnold Arboretum dis- into elongated fruits with numerous bright red, play incredible floral diversity. Magnolia berrylike fruitlets. Winter will reveal exfoliating Tmacrophylla’s huge waxy blooms open bark resembling that of climbing hydrangea. twice, partly closing in between for an over- Evolutionary biologists (including Arboretum night sex change. Helwingia japonica sprouts director Ned Friedman) have discovered that tiny green umbels in the center of otherwise Schisandra and the other Austrobaileyales can unremarkable leaves. Davidia involucrata for- offer insight into many key events in the his- goes petals entirely, but shelters its reproductive tory of flowering plants. Aspects of Schisandra’s organs with massive white bracts. Even wild vascular system may represent an early step in Viola sororia, flagging down bees with its iconic the development of vessels, the structures that violets, surreptitiously sends out discrete, self- allow most flowering plants to rapidly trans- pollinating flowers underground. port water and ecologically dominate hot and With all this bizarre and beautiful reproduc- dry habitats. Schisandra also retains a relatively tion going on, most of us overlook the most simple anatomy during its haploid stage, with evolutionarily distinctive flowering plant in only four nuclei and one developmental module the collection: Schisandra chinensis. An unas- in each female gametophyte (almost all flower- ing plants have eight nuclei and two modules). suming woody vine, it represents a unique and The endosperm of Schisandra seeds conse- ancient lineage that parted ways with most other quently contains only one complement of genes flowering plants at least as far back as the early from each of its parents, while most flowering Cretaceous, before even “living fossils” like plants acquire an additional copy of their moms’ Magnolia. -

NPN Winter 2014.Spub
NATIVE PLANT NEWS the newsletter of the North Carolina Native Plant Society VOLUME 12, ISSUE 1 ISSN: 2151-2159 WINTER 2014 NCNPS Spring Trip 2014: May 16–18: Green Swamp & Lake BE PREPARED for an amazing Spring Trip in 2014, when we will visit some of North Carolina’s most fascinating habitats. Friday afternoon early arrivals will do some highway botanizing along Highways 130 and 17. The Friday night program will be at Lake Waccamaw State Park (1866 State Park Drive, Lake Waccamaw NC 28450), where Park Superintendent Toby Hall will introduce us to the soils and plants of Lake Waccamaw. Saturday David McAdoo, Mark Rose, Angie Carl, and Robert Thornhill will lead us exploring the Green Swamp, which The Nature Conservancy has called “a place unlike any other”. Among the plants we’re likely to see are Venus Fly-trap (Dionaea muscipula), three species of (each) pitcherplants, sundews, and Calopogon orchids; bladderworts, Goldencrest (Lophiola aurea), two species of Cleistes orchid, and much more. It will be a day to remember! The Saturday evening dinner and native plant auction will be at the NC Museum of Forestry (415 S. Madison Street, Whiteville NC 28472) in Whiteville. So start potting up those plants soon! Sunday morning we will visit Lake Waccamaw (1866 State Park Drive, Lake Waccamaw NC 28450), where the park ranger will give us a presentation and then we’ll botanize around the lake. We will wrap up by 1:00 p.m. We’ll be staying at the EconoLodge Hotel in Whiteville, at 503 J. L. Powell Blvd. -

SWAP 2015 Report
STATE WILDLIFE ACTION PLAN September 2015 GEORGIA DEPARTMENT OF NATURAL RESOURCES WILDLIFE RESOURCES DIVISION Georgia State Wildlife Action Plan 2015 Recommended reference: Georgia Department of Natural Resources. 2015. Georgia State Wildlife Action Plan. Social Circle, GA: Georgia Department of Natural Resources. Recommended reference for appendices: Author, A.A., & Author, B.B. Year. Title of Appendix. In Georgia State Wildlife Action Plan (pages of appendix). Social Circle, GA: Georgia Department of Natural Resources. Cover photo credit & description: Photo by Shan Cammack, Georgia Department of Natural Resources Interagency Burn Team in Action! Growing season burn on May 7, 2015 at The Nature Conservancy’s Broxton Rocks Preserve. Zach Wood of The Orianne Society conducting ignition. i Table&of&Contents& Acknowledgements ............................................................................................................ iv! Executive Summary ............................................................................................................ x! I. Introduction and Purpose ................................................................................................. 1! A Plan to Protect Georgia’s Biological Diversity ....................................................... 1! Essential Elements of a State Wildlife Action Plan .................................................... 2! Species of Greatest Conservation Need ...................................................................... 3! Scales of Biological Diversity -

Female Gamete Competition in an Ancient Angiosperm Lineage
Female gamete competition in an ancient angiosperm lineage Julien B. Bacheliera,b,c and William E. Friedmana,b,c,1 aDepartment of Ecology and Evolutionary Biology, University of Colorado, Boulder, CO 80309; bDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; and cArnold Arboretum, Harvard University, Boston, MA 02131 Edited* by Peter H. Raven, Missouri Botanical Garden, St. Louis, MO, and approved May 19, 2011 (received for review March 23, 2011) In Trimenia moorei, an extant member of the ancient angiosperm ly diversification of floral morphology and anatomy are widely clade Austrobaileyales, we found a remarkable pattern of female viewed to have led to increased levels of pollen reception and gametophyte (egg-producing structure) development that strik- hence, male–male competition (4). Collectively, the evolution of ingly resembles that of pollen tubes and their intrasexual compe- the carpel, extragynoecial compitum, transmitting tissue, callose tition within the maternal pollen tube transmitting tissues of most plugs, and insect pollination seems to have resulted in signifi- flowers. In contrast with most other flowering plants, in Trimenia, cantly enhanced levels of prefertilization male competition and multiple female gametophytes are initiated at the base (chalazal maternal choice, and thus may have played a major role in the end) of each ovule. Female gametophytes grow from their tips and early diversification of angiosperms (4–9). compete over hundreds of micrometers to reach the apex of the Despite all of the attention paid to prefertilization mecha- nucellus and the site of fertilization. Here, the successful female nisms of male competition and female choice, there has never gametophyte will mate with a pollen tube to produce an embryo been any discussion of the possibility that female gametophytes and an endosperm. -

Evaluation of Neuroactive Effects of Ethanol Extract of Schisandra
Chinese Journal of Natural Chinese Journal of Natural Medicines 2018, 16(12): 09160925 Medicines Evaluation of neuroactive effects of ethanol extract of Schisandra chinensis, Schisandrin, and Schisandrin B and determination of underlying mechanisms by zebrafish behavioral profiling WANG Jia-Wei1Δ, LIANG Feng-Yin2Δ, OUYANG Xiang-Shuo1, LI Pei-Bo1, PEI Zhong2*, SU Wei-Wei 1* 1 Guangdong Engineering and Technology Research Centre for Quality and Efficacy Re-evaluation of Post-marketed TCM, Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China; 2 Department of Neurology, National Key Clinical Department and Key Discipline of Neurology, Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Major Neurological Diseases, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, China Available online 20 Dec., 2018 [ABSTRACT] Schisandra chinensis, a traditional Chinese medicine (TCM), has been used to treat sleep disorders. Zebrafish sleep/wake behavioral profiling provides a high-throughput platform to screen chemicals, but has never been used to study extracts and components from TCM. In the present study, the ethanol extract of Schisandra chinensis and its two main lignin components, schisan- drin and schisandrin B, were studied in zebrafish. We found that the ethanol extract had bidirectional improvement in rest and activity in zebrafish. Schisandrin and schisandrin B were both sedative and active components. We predicted that schisandrin was related to serotonin pathway and the enthanol extract of Schisandra chinensis was related to seoronin and domapine pathways using a database of zebrafish behaviors. These predictions were confirmed in experiments using Caenorhabditis elegans. In conclusion, zebrafish behavior profiling could be used as a high-throughput platform to screen neuroactive effects and predict molecular pathways of extracts and components from TCM.