Furstbruce1982.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Source to Surface Model of Monogenetic Volcanism: a Critical Review
Downloaded from http://sp.lyellcollection.org/ by guest on September 28, 2021 Source to surface model of monogenetic volcanism: a critical review I. E. M. SMITH1 &K.NE´ METH2* 1School of Environment, University of Auckland, Auckland, New Zealand 2Volcanic Risk Solutions, Massey University, Palmerston North 4442, New Zealand *Correspondence: [email protected] Abstract: Small-scale volcanic systems are the most widespread type of volcanism on Earth and occur in all of the main tectonic settings. Most commonly, these systems erupt basaltic magmas within a wide compositional range from strongly silica undersaturated to saturated and oversatu- rated; less commonly, the spectrum includes more siliceous compositions. Small-scale volcanic systems are commonly monogenetic in the sense that they are represented at the Earth’s surface by fields of small volcanoes, each the product of a temporally restricted eruption of a composition- ally distinct batch of magma, and this is in contrast to polygenetic systems characterized by rela- tively large edifices built by multiple eruptions over longer periods of time involving magmas with diverse origins. Eruption styles of small-scale volcanoes range from pyroclastic to effusive, and are strongly controlled by the relative influence of the characteristics of the magmatic system and the surface environment. Gold Open Access: This article is published under the terms of the CC-BY 3.0 license. Small-scale basaltic magmatic systems characteris- hazards associated with eruptions, and this is tically occur at the Earth’s surface as fields of small particularly true where volcanic fields are in close monogenetic volcanoes. These volcanoes are the proximity to population centres. -

National Weather Service High Wind Warning
National Weather Service Text Product Display https://forecast.weather.gov/product.php?site=HFO&issuedby=HFO&pr... National Weather Service Weather Forecast Office Honolulu, HI Non-Precipitation Warnings / Watches / Advisories Issued by NWS Honolulu, HI Home | Current Version | Previous Version | Text Only | Print | Product List | Glossary On Versions: 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 000 WWHW70 PHFO 071330 NPWHFO URGENT - WEATHER MESSAGE National Weather Service Honolulu HI 330 AM HST Sat Mar 7 2020 ...HIGH WIND WARNING FOR LEEWARD WEST MAUI LANAI KAHOOLAWE AND THE LEEWARD KOHALA DISTRICT UNTIL 6 PM HST THIS EVENING... ...WIND ADVISORY FOR ALL HAWAIIAN ISLANDS UNTIL 6 PM HST THIS EVENING... .High pressure far north of the islands will support strong to locally damaging trade winds today, with winds gradually weakening tonight and Sunday. HIZ014>016-018-026-080400- /O.CON.PHFO.HW.W.0003.000000T0000Z-200308T0400Z/ Lanai Makai-Lanai Mauka-Kahoolawe-Maui Leeward West-Kohala- Including the cities of Manele, Lanai City, Lahaina, Kaanapali, and Waikoloa 330 AM HST Sat Mar 7 2020 ...HIGH WIND WARNING REMAINS IN EFFECT UNTIL 6 PM HST THIS EVENING... * WHAT...Northeast winds 30 to 40 mph with localized gusts over 60 mph. * WHERE...Leeward West Maui, Lanai, Kahoolawe and the Leeward Kohala District on the Big Island. * WHEN...Until 6 PM HST this evening. * IMPACTS...Damaging winds will blow down trees and power lines. Sporadic power outages can be expected. Travel will be difficult, especially for high profile vehicles. PRECAUTIONARY/PREPAREDNESS ACTIONS... Motorists, especially those in high profile vehicles, are urged to drive with extreme caution. -

31 a Preliminary Study Of
31 A PRELIMINARY STUDY OF THE: TERTIARY VOLCANIC AND SEDIMENTARY ROCKS, GÜMELE, ESKİŞEHİR Eskişehir, Gümele Çevresindeki Tersiyer Volkanik ve Sedimanter Kayaçlarda Bir Ön Çalışma Taylan Lünel Middle East Technical University, Department of Geological Engineering Ankara ÖZ. — Seyitgazi-Eskişehir antiklinoriumu'nun çok fazla deforme olmuş ve metamorfizmaya uğramış kayaçlarının kuzey-kuzeybatısında bulunan sedimanter ve volkanik kayaçlar incelenmiştir. Karasal ve gölsel fasiyesde meydana gelen Tersi- yer sedimanter kayaçlar Güney Eskişehir küvetinde olunmuşlardır. Karasal fasiye- si meydana getiren kayaç birimlerini kaba kumtaşları, kumtaşları, bitki kalıntıları ihtiva eden kil ve marnlar ve serpantinit blokları taşıyan bazal konglomerası teşkil etmektedir. Gölsel fasiyes ise genellikle killi ve tüflü kalkerler, kalkerler, marnlar, kon- glomeralar ve tüflerden meydana gelmiştir. Küvetteki en eski sedimanlar ve piroklas- tikler Alt Miosen'de oluşmuşlardır. Yataya yakın konumlanmış bazik-intermediyar lav akıntıları Pliosen yaşlı olup Altüst Neojen sedimantasyon kesikliğinde meyda- na gelmiştir. Üst Neojen sedimanter kayaçları intermediyar-basaltik volkaniklerin üzerinde ince bandlar şeklinde bulunurlar. Bu birim marn ve kalkerlerden meyda- na gelmiştir. Alt Miosen’de asid volkanik faaliyetler neticesinde meydana gelen sil- lar (unweldd tuffs) oligomikt konglomeralardan evvel teşekkül etmiştir. Bu volkanik aktitivite muhtemelen kesikli ve kısıtlı olarak devam etmiş ve tüflü kalkerleri mey- dana getirmiştir. Pliosen yaşlı bazik-intermediyar -

Precontact Vegetation and Soil Nutrient Status in the Shadow of Kohala Volcano, Hawaii ⁎ Oliver A
Geomorphology 89 (2007) 70–83 www.elsevier.com/locate/geomorph Precontact vegetation and soil nutrient status in the shadow of Kohala Volcano, Hawaii ⁎ Oliver A. Chadwick a, , Eugene F. Kelly b, Sara C. Hotchkiss c, Peter M. Vitousek d a Department of Geography, University of California, Santa Barbara, CA 93106, USA b Department of Crop and Soil Science, Colorado State University, Fort Collins, CO 80523, USA c Department of Botany, University of Wisconsin, Madison, WI 53706, USA d Department of Biological Sciences, Stanford University, Stanford, CA 94305, USA Received 13 January 2005; received in revised form 25 July 2006; accepted 25 July 2006 Available online 2 October 2006 Abstract Humans colonized Hawaii about 1200 years ago and have progressively modified vegetation, particularly in mesic to dry 13 tropical forests. We use δ C to evaluate the contribution of C3 and C4 plants to deep soil organic matter to reconstruct pre-human contact vegetation patterns along a wet to dry climate transect on Kohala Mountain, Hawaii Island. Precontact vegetation assemblages fall into three distinct zones: a wet C3 dominated closed canopy forest where annual rainfall is N2000 mm, a dry C4 dominated grassland with annual rainfall b500 mm, and a broad transition zone between these communities characterized by either C3 trees with higher water-use efficiency than the rainforest trees or C3 trees with a small amount of C4 grasses intermixed. The likelihood of C4 grass understory decreases with increasing rainfall. We show that the total concentration of rock-derived nutrients in the b2-mm soil fraction differs in each of these vegetation zones. -

THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution
VOLCANISM IN HAWAII Chapter 1 - .-............,. THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution By David A. Clague and G. Brent Dalrymple ABSTRACT chain, the near-fixity of the hot spot, the chemistry and timing of The Hawaiian-Emperor volcanic chain stretches nearly the eruptions from individual volcanoes, and the detailed geom 6,000 km across the North Pacific Ocean and consists of at least etry of volcanism. None of the geophysical hypotheses pro t 07 individual volcanoes with a total volume of about 1 million posed to date are fully satisfactory. However, the existence of km3• The chain is age progressive with still-active volcanoes at the Hawaiian ewell suggests that hot spots are indeed hot. In the southeast end and 80-75-Ma volcanoes at the northwest addition, both geophysical and geochemical hypotheses suggest end. The bend between the Hawaiian and .Emperor Chains that primitive undegassed mantle material ascends beneath reflects a major change in Pacific plate motion at 43.1 ± 1.4 Ma Hawaii. Petrologic models suggest that this primitive material and probably was caused by collision of the Indian subcontinent reacts with the ocean lithosphere to produce the compositional into Eurasia and the resulting reorganization of oceanic spread range of Hawaiian lava. ing centers and initiation of subduction zones in the western Pacific. The volcanoes of the chain were erupted onto the floor of the Pacific Ocean without regard for the age or preexisting INTRODUCTION structure of the ocean crust. Hawaiian volcanoes erupt lava of distinct chemical com The Hawaiian Islands; the seamounts, hanks, and islands of positions during four major stages in their evolution and the Hawaiian Ridge; and the chain of Emperor Seamounts form an growth. -

Geology, Geochemistry and Earthquake History of Lō`Ihi Seamount, Hawai`I
INVITED REVIEW Geology, Geochemistry and Earthquake History of Lō`ihi Seamount, Hawai`i Michael O. Garcia1*, Jackie Caplan-Auerbach2, Eric H. De Carlo3, M.D. Kurz4 and N. Becker1 1Department of Geology and Geophysics, University of Hawai`i, Honolulu, HI, USA 2U.S.G.S., Alaska Volcano Observatory, Anchorage, AK, USA 3Department of Oceanography, University of Hawai`i, Honolulu, HI, USA 4Department of Chemistry, Woods Hole Oceanographic Institution, Woods Hole, MA, USA *Corresponding author: Tel.: 001-808-956-6641, FAX: 001-808-956-5521; email: [email protected] Key words: Loihi, seamount, Hawaii, petrology, geochemistry, earthquakes Abstract A half century of investigations are summarized here on the youngest Hawaiian volcano, Lō`ihi Seamount. It was discovered in 1952 following an earthquake swarm. Surveying in 1954 determined it has an elongate shape, which is the meaning of its Hawaiian name. Lō`ihi was mostly forgotten until two earthquake swarms in the 1970’s led to a dredging expedition in 1978, which recovered young lavas. This led to numerous expeditions to investigate the geology, geophysics, and geochemistry of this active volcano. Geophysical monitoring, including a real- time submarine observatory that continuously monitored Lō`ihi’s seismic activity for three months, captured some of the volcano’s earthquake swarms. The 1996 swarm, the largest recorded in Hawai`i, was preceded by at least one eruption and accompanied by the formation of a ~300-m deep pit crater, renewing interest in this submarine volcano. Seismic and petrologic data indicate that magma was stored in a ~8-9 km deep reservoir prior to the 1996 eruption. -
1 Appendix 3. Modes of Samples in the San Francisco Mountain
Appendix 3. Modes of Samples in the San Francisco Mountain Volcanic System, and Selected Basalts from the Eastern San Francisco Volcanic Field. Minerals in Volume Percent. 1 2 3 4 5 6 7 Sample 3823A 2A06 2812A 2031A 2031.B 3732J DC04B Map Name basalt basalt basalt basalt basalt andesite andesite TAS Name basalt basalt basalt basalt basalt mugearite mugearite Field flow flow flow flow flow flow flow SiO2 48.7 48.9 49.7 50.4 51.0 52.2 53.0 Plagioclase 14.6, An69 8.3, An70 25.6,An67 25.5,An71 20.2,An74 29, An64 32.0,An62 Olivine 11.2, Fo83 10.9,Fo84 8.1, Fo73 7.0, Fo77 3.9, Fo76 3, Fo75 6.6, Fo75 Clinopyroxene 4.3 4.3 4.6 1.8 1.3 0 trace Opaque Oxide 4.1 0.2 0.5 1.6 0.3 1 2.4 Matrix 65.8 76.3 61.2 64.1 74.4 67 59.0 Points 1165 1173 1504 1464 1347 827 1549 Matrix intergran- inter- intergran- intergran- inter- microcrys- inter- Texture ular sertal ular ular sertal talline sertal MF1959 Symbol Qa1 Qa1 I-1663 Symbol Qao Qao 8 9 10 11 12 13 14 Sample 3732K 3732Z 3732Y 2705D 3831A 3729Q 3707 Map Name andesite andesite andesite andesite andesite andesite andesite TAS Name mugearite mugearite mugearite mugearite mugearite mugearite mugearite Field flow flow dike flow flow flow flow SiO2 53.4 53.7 53.8 55.2 55.3 56.0 56.6 Plagioclase 24.8,An64 15.3,An61 30.2,An61 42.1,An52 27.0,An62 29.8,An57 41.2,An57 Olivine 2.8, Fo69 3.1, Fo65 4.1 5.6 4.3, Fo62 3.7 Fo57 2.5, Fo58 Clinopyroxene 0.2 0.5 1.5 2.1 0.8 3.0 1.1 Orthopyroxene - - 0.5 - - 0.2 - Opaque Oxide 0.3 0.8 3.0 1.0 0.6 2.1 1.4 Matrix 71.8 80.3 60.7 49.2 67.2 61.1 53.8 Points 1196 1785 854 1844 1428 1029 1540 Matrix hyalo- inter- inter- hyalo- hyalo- hyalo- micro- Texture pilitic granular sertal ophitic pilitic ophitic crystalline MF1959 Symbol Qa1 Qa1 Qai Qa1 Qa2 Qa2 Qa2 I-1663 Symbol Qao Qao Qai Qao Qay Qay Qay Notes: 1. -

Direct Derivation of Benmoreite to Phonolite
EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES Didier LAPORTE, Sarah LAMBART, Pierre SCHIANO, and Luisa OTTOLINI SUPPLEMENTARY MATERIAL 1. Experimental and analytical techniques 1.1 Starting materials. The starting material used to prepare our two fertile mantle compositions was a fresh spinel lherzolite xenolith (Bri3) from Mont Briançon volcano, French Massif Central. Because a fine grain size is critical to attain chemical equilibrium, lherzolite Bri3 was first pulverized in a micronizing mill for 30 min to reduce its grain size to 2-4 µm. The powder was then fired 5 hours at 900°C in a CO2/H2 atmosphere with gas flow rates adjusted to yield an oxygen fugacity between the magnetite-wüstite and the iron-wüstite -15.91 buffers (fO2 = 10 bar). As Bri3 contains only 100 ppm K2O, small amounts of synthetic basalt B2 were added to Bri3 to prepare the fertile mantle compositions MBK (410 ppm K2O) and MBK+ (930 ppm K2O; compositions Bri3, B2, MBK and MBK+ are given in Table 1). Mixtures of Bri3 and B2 were homogenized by grinding in an agate mortar for one hour. The starting material for reversal experiments MBK+13 and MBK+14 was a mixture of 80.16 % MBK+ and 19.84 % phonolite gel +6 (Table 1). Phonolite gel +6 was prepared using the gel method (Luth and Ingamells, 1965), and then fired at the same T and fO2 as lherzolite Bri3. Gel +6 matches well the target composition (that is, the glass in partial melting experiment MBK+6), except that it is ≈ 10 % richer in Na2O. -

Geologic Map of the State of Hawai 'I
Geologic Map of the State of Hawai‘i By David R. Sherrod, John M. Sinton, Sarah E. Watkins, and Kelly M. Brunt Open-File Report 2007–1089 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested citation: Sherrod, D.R., Sinton, J.M., Watkins, S.E., and Brunt, K.M., 2007, Geologic Map of the State of Hawai`i: U.S. Geological Survey Open-File Report 2007-1089, 83 p., 8 plates, scales 1:100,000 and 1:250,000, with GIS database Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. ii Geologic Map of the State of Hawai‘i By David R. Sherrod, John M. Sinton, Sarah E. Watkins, and Kelly M. Brunt About this map Sources of mapping, methods of This geologic map and its digital databases present compilation, origin of stratigraphic the geology of the eight major islands of the State of names, and divisions of the geologic Hawai‘i. -
Maunaloa/Current/Longterm.Html K!Lauea – the Most Active Volcano on Earth K!Lauea Structure
!"#$%&'()'*$+$,-, !"#$%"& '()*%"+, -*./&0%1(2343(5"6"+7+ 8#9*)"/& :/*;0,& .(/$#$ 0$1%$'.2$ 4'5(6'78' "29$6$:2' ;(#<$%(2" *1$#!#$, ."#$12$ 0$1%$'3($ 3#-,/, =(#<$%,<'>:$?2" 0$1%$'3($'$%&'."#$12$@''''' A "/,2#&'#$;$"'(%#B *1$#!#$,@'''''''''''''''''''''''''''''' A 9(":"/,2#& (%#B .(/$#$ $%&'0$1%$'.2$@'''' A "/,2#&'C'9(":"/,2#& DEEFEEE'G HEEFEEE' B6"'$?(F'$##'4' ;(#<$%(2"'+262' $<:,;2'5%(+'I'$62J' (%#B'D',"'2K:,%<:8 LDMEFEEE'B6"'$?(' ."#$12$F'0N'3($F'0N' .2$'O'*1$#!#$, +262'$<:,;2'"/,2#&' ;(#<$%(2"F'+/,#2' .(/$#$ +$"',%':/2' 9(":A"/,2#&'":$?2 P!/$#$ Q"/ < 1*./(=**> ?@+,>#0%%(+#()0&0/% A",$*#"B$(0&("BCD(EFGH P!/$#$ Q"/'62&2),%2& I3B)*%&(,0/&"+#B1()*/0(&@"#(*#0( %*./,0(9*/(&@0("%@(=0$%(*#(&@0(J+K( 8%B"#$ I ?@*%0(+#(&@0(#*/&@("/0(LEMMDMMM(1/%( *B$("#$(N/*="=B1($0/+O0$(9/*)(A".#"( P0"(Q#*(B*#K0/(,"BB0$(:!@"B" 3%@R I ?@*%0(*#(A".#"(!*"("#$(P"B".0" "/0().,@(1*.#K0/(QSEMIEH(>"R("#$( N/*="=1 $0/+O0$(9/*)(0TNB*%+O0( 0/.N&+*#%(*#(A".#"(!*"("#$(P"B".0"C(( Q"/':/,<R%2""',%'S2:26" T,?'!"#$%&'U;(#1:,(% V/2':+('(#&2":'T,?'!"#$%&' ;(#<$%(2"'$62'.(/$#$ 5.(8'$%&' $'"1WS$6,%2';(#<$%('<$##2&' 0!/1R(%$ 508 T(:/'+262'96(W$W#B'962"2%:' 4EEFEEE'B6"'$?( 0!/1R(%$ G :/2'S,"",%?'*$+$,,$%';(#<$%( U"/,+"(0&("BCD(EFFM -B"K.0 "#$(A**/0D(EFFE IM4'G 4EE'R$':/(#2,,:,< $%&'$#R$#,< W$"$#:" QW(6:2&'X962A"/,2#&Y'(6'W16,2&':6$%",:,(%$#'9(":"/,2#&Z T,?'!"#$%&'U;(#1:,(% TB'MEEFEEE'B2$6"'$?( A!@.>*#" 6"%(0T&+#,&(QVR( P*@"B"D(A".#"(P0"D("#$(5."B!B"+ 60/0(",&+O0(%@+0B$% P*@"B" @"$(.#$0/K*#0("()";*/(B"#$%B+$0 :/*="=B1(A".#"(!*"(6"%("B%*(",&+O0("&(&@+%(&+)0 T,?'!"#$%&'U;(#1:,(% TB'DEEFEEE'B2$6"'$?( -
Magmas Parental to the Chassigny Meteorite: New Considerations
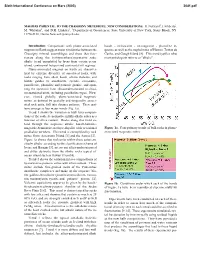
Sixth International Conference on Mars (2003) 3041.pdf MAGMAS PARENTAL TO THE CHASSIGNY METEORITE: NEW CONSIDERATIONS. H. Nekvasil1, J. Filiberto1, M. Whitaker1, and D.H. Lindsley1, 1Department of Geosciences, State University of New York, Stony Brook, NY 11794-2100, [email protected]. Introduction: Comparisons with plume-associated basalt - ne-hawaiite - ne-mugearite - phonolite se- magmas on Earth suggest major similarities between the quence as well as the nephelinites of Hawaii, Tristan da Chassigny mineral assemblages and those that frac- Cunha, and Gough Island [8]. This trend typifies what tionate along the hy(hypersthene)-normative sodic most petrologists refer to as "alkalic". alkalic trend exemplified by lavas from certain ocean island, continental hotspot and continental rift regimes. Plume-associated magmas on Earth are character- ized by extreme diversity of associated rocks, with rocks ranging from alkali basalt, olivine tholeiite, and biotite gabbro to anorthosite, syenite, comendite, pantellerite, phonolite and potassic granite, and span- ning the spectrum from silica-undersaturated to silica- oversaturated units, including peralkaline types. How- ever, viewed globally, plume-associated magmatic suites, as defined by spatially and temporally associ- ated rock units, fall into distinct patterns. These pat- terns emerge as four major trends (Fig. 1a). Trend 1 shows the variation in bulk lava composi- tions of the sodic hy-normative mildly alkalic suites as a function of silica content. Rocks along this trend ex- tend through the sequence alkalic basalt-hawaiite- mugearite-benmoreite-trachyte-rhyolite with occasional Figure 1a. Four primary trends of bulk rocks in plume- peralkaline members. This trend is exemplified by rock associated magmatic suites. -

Petrology, Mineralogy, and Geochemistry of the East Molokai Volcanic Series, Hawaii
Petrology, Mineralogy, and Geochemistry of the East Molokai Volcanic Series, Hawaii GEOLOGICAL SURVEY PROFESSIONAL PAPER 961 Petrology, Mineralogy, and Geochemistry of the East Molokai Volcanic Series, Hawaii By MELVIN H. BEESON GEOLOGICAL SURVEY PROFESSIONAL PAPER 961 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON: 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress catalog-card No. 76-12795 For ~ale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock Number 024-001-02810-7 CONTENTS Page Page 1\bstract__________________________________________________ 1 Phenocryst-free compositions ------------------------------ 14 Introduction ---------------------------------------------- 1 Mineralogy ____ __ __ __ __ ______ __ ________ __ __________ ______ 21 Previous investigations------------------------------------ 3 Compositional variation of olivine______________________ 21 Purpose and scope ---------------------------------------- 4 Compositional variation of clinopyroxene ______________ 25 1\nalytical procedures ------------------------------------ 4 Compositional variation of plagioclase__________________ 27 Stratigraphy and petrology-------------------------------- 6 Compositional variation of opaque minerals ____________ 28 Whole-rock compositions ---------------------------------- 11 Summary ------------------------------------------------ 30 References________________________________________________ 33