Petrology, Mineralogy, and Geochemistry of the East Molokai Volcanic Series, Hawaii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
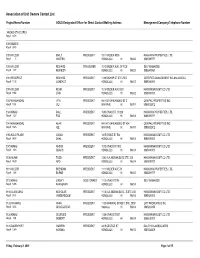
Association of Unit Owners Contact List
Association of Unit Owners Contact List Project Name/Number AOUO Designated Officer for Direct Contact/Mailing Address Management Company/Telephone Number `AKOKO AT HO`OPILI Reg.# 8073 1001 QUEEN Reg.# 7675 1001 WILDER EMILY PRESIDENT 1001 WILDER #305 HAWAIIAN PROPERTIES, LTD. Reg.# 5 WATERS HONOLULU HI 96822 8085399777 1010 WILDER RICHARD TREASURER 1010 WILDER AVE, OFFICE SELF MANAGED Reg.# 377 KENNEDY HONOLULU HI 96822 8085241961 1011 PROSPECT RICHARD PRESIDENT 1188 BISHOP ST STE 2503 CERTIFIED MANAGEMENT INC dba ASSOCI Reg.# 1130 CONRADT HONOLULU HI 96813 8088360911 1015 WILDER KEVIN PRESIDENT 1015 WILDER AVE #201 HAWAIIANA MGMT CO LTD Reg.# 1960 LIMA HONOLULU HI 96822 8085939100 1037 KAHUAMOKU VITA PRESIDENT 94-1037 KAHUAMOKU ST 3 CEN PAC PROPERTIES INC Reg.# 1551 VILI WAIPAHU HI 96797 8085932902 1040 KINAU PAUL PRESIDENT 1040 KINAU ST., #1206 HAWAIIAN PROPERTIES, LTD. Reg.# 527 FOX HONOLULU HI 96814 8085399777 1041 KAHUAMOKU ALAN PRESIDENT 94-1041 KAHUAMOKU ST 404 CEN PAC PROPERTIES INC Reg.# 1623 IGE WAIPAHU HI 96797 8085932902 1054 KALO PLACE JUANA PRESIDENT 1415 S KING ST 504 HAWAIIANA MGMT CO LTD Reg.# 5450 DAHL HONOLULU HI 96814 8085939100 1073 KINAU ANSON PRESIDENT 1073 KINAU ST 1003 HAWAIIANA MGMT CO LTD Reg.# 616 QUACH HONOLULU HI 96814 8085939100 1108 AUAHI TODD PRESIDENT 1240 ALA MOANA BLVD STE. 200 HAWAIIANA MGMT CO LTD Reg.# 7429 APO HONOLULU HI 96814 8085939100 1111 WILDER BRENDAN PRESIDENT 1111 WILDER AVE 7A HAWAIIAN PROPERTIES, LTD. Reg.# 228 BURNS HONOLULU HI 96822 8085399777 1112 KINAU LINDA Y SOLE OWNER 1112 KINAU ST PH SELF MANAGED Reg.# 1295 NAKAGAWA HONOLULU HI 96814 1118 ALA MOANA NICHOLAS PRESIDENT 1118 ALA MOANA BLVD., SUITE 200 HAWAIIANA MGMT CO LTD Reg.# 7431 VANDERBOOM HONOLULU HI 96814 8085939100 1133 WAIMANU ANNA PRESIDENT 1133 WAIMANU STREET, STE. -

Pele's Tears by D.A
Issues in Earth Science “Eww, There’s Some Geology in my Fiction!” Issue 9, May-June 2018 Teacher Resources Can Alana use clues from geology to clear her name after she's accused of stealing a volcanic stone on her tour of Mauna Loa in Hawaiʻi? Pele's Tears by D.A. Xiaolin Spires Divya stood up next to me and opened the window, while I fanned myself with a glossy book I’d brought to read on the bus. It had a photo of sapphires and rubies below the title, Rocks, Gems and Crystals- Geoscience for Amateurs. It was my third time reading it, but I couldn’t Pele's Tears — D.A. Xiaolin Spires Issues in Earth Science focus. It was hot in May at the Visitor Center at Kīlauea. My thighs were sticking to the school bus seats. Parked in front of Kīlauea Visitor Center, my classmates and I couldn’t wait to get out and check out Hawaiʻi’s famous Big Island volcanoes. Divya wiped sweat from her forehead with her sleeve. I took a sip of water from the bottle I carried in my backpack. I reached into my pocket to compare my Pele’s tear to a photo of a jet black arrowhead I saw in my book. It occurred to me that they looked a bit similar. “What’s that?” asked Divya. She pointed at my fist. I passed my Pele’s tear to Divya. She felt the weight of it in her palm. Obsidian black, long and shiny, it glimmered in the sun. -

Topographic History of the Maui Nui Complex, Hawai'i, and Its Implications for Biogeography1
Topographic History ofthe Maui Nui Complex, Hawai'i, and Its Implications for Biogeography 1 Jonathan Paul Price 2,4 and Deborah Elliott-Fisk3 Abstract: The Maui Nui complex of the Hawaiian Islands consists of the islands of Maui, Moloka'i, Lana'i, and Kaho'olawe, which were connected as a single landmass in the past. Aspects of volcanic landform construction, island subsi dence, and erosion were modeled to reconstruct the physical history of this complex. This model estimates the timing, duration, and topographic attributes of different island configurations by accounting for volcano growth and subsi dence, changes in sea level, and geomorphological processes. The model indi cates that Maui Nui was a single landmass that reached its maximum areal extent around 1.2 Ma, when it was larger than the current island of Hawai'i. As subsi dence ensued, the island divided during high sea stands of interglacial periods starting around 0.6 Ma; however during lower sea stands of glacial periods, islands reunited. The net effect is that the Maui Nui complex was a single large landmass for more than 75% of its history and included a high proportion of lowland area compared with the contemporary landscape. Because the Hawaiian Archipelago is an isolated system where most of the biota is a result of in situ evolution, landscape history is an important detertninant of biogeographic pat terns. Maui Nui's historical landscape contrasts sharply with the current land scape but is equally relevant to biogeographical analyses. THE HAWAIIAN ISLANDS present an ideal logic histories that can be reconstructed more setting in which to weigh the relative influ easily and accurately than in most regions. -

National Weather Service High Wind Warning
National Weather Service Text Product Display https://forecast.weather.gov/product.php?site=HFO&issuedby=HFO&pr... National Weather Service Weather Forecast Office Honolulu, HI Non-Precipitation Warnings / Watches / Advisories Issued by NWS Honolulu, HI Home | Current Version | Previous Version | Text Only | Print | Product List | Glossary On Versions: 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 000 WWHW70 PHFO 071330 NPWHFO URGENT - WEATHER MESSAGE National Weather Service Honolulu HI 330 AM HST Sat Mar 7 2020 ...HIGH WIND WARNING FOR LEEWARD WEST MAUI LANAI KAHOOLAWE AND THE LEEWARD KOHALA DISTRICT UNTIL 6 PM HST THIS EVENING... ...WIND ADVISORY FOR ALL HAWAIIAN ISLANDS UNTIL 6 PM HST THIS EVENING... .High pressure far north of the islands will support strong to locally damaging trade winds today, with winds gradually weakening tonight and Sunday. HIZ014>016-018-026-080400- /O.CON.PHFO.HW.W.0003.000000T0000Z-200308T0400Z/ Lanai Makai-Lanai Mauka-Kahoolawe-Maui Leeward West-Kohala- Including the cities of Manele, Lanai City, Lahaina, Kaanapali, and Waikoloa 330 AM HST Sat Mar 7 2020 ...HIGH WIND WARNING REMAINS IN EFFECT UNTIL 6 PM HST THIS EVENING... * WHAT...Northeast winds 30 to 40 mph with localized gusts over 60 mph. * WHERE...Leeward West Maui, Lanai, Kahoolawe and the Leeward Kohala District on the Big Island. * WHEN...Until 6 PM HST this evening. * IMPACTS...Damaging winds will blow down trees and power lines. Sporadic power outages can be expected. Travel will be difficult, especially for high profile vehicles. PRECAUTIONARY/PREPAREDNESS ACTIONS... Motorists, especially those in high profile vehicles, are urged to drive with extreme caution. -

Conversing with Pelehonuamea: a Workshop Combining 1,000+ Years of Traditional Hawaiian Knowledge with 200 Years of Scientific Thought on Kīlauea Volcanism
Conversing with Pelehonuamea: A Workshop Combining 1,000+ Years of Traditional Hawaiian Knowledge with 200 Years of Scientific Thought on Kīlauea Volcanism Open-File Report 2017–1043 Version 1.1, June 2017 U.S. Department of the Interior U.S. Geological Survey Conversing with Pelehonuamea: A Workshop Combining 1,000+ Years of Traditional Hawaiian Knowledge with 200 Years of Scientific Thought on Kīlauea Volcanism Compiled and Edited by James P. Kauahikaua and Janet L. Babb Open-File Report 2017–1043 Version 1.1, June 2017 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior RYAN K. ZINKE, Secretary U.S. Geological Survey William H. Werkheiser, Acting Director U.S. Geological Survey, Reston, Virginia: 2017 First release: 2017 Revised: June 2017 (ver. 1.1) For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov/ or call 1–888–ASK–USGS (1–888–275–8747). For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod/. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner. Suggested citation: Kauahikaua, J.P., and Babb, J.L., comps. and eds., Conversing with Pelehonuamea—A workshop combining 1,000+ years of traditional Hawaiian knowledge with 200 years of scientific thought on Kīlauea volcanism (ver. -

Hawaiian Volcanoes: from Source to Surface Site Waikolao, Hawaii 20 - 24 August 2012
AGU Chapman Conference on Hawaiian Volcanoes: From Source to Surface Site Waikolao, Hawaii 20 - 24 August 2012 Conveners Michael Poland, USGS – Hawaiian Volcano Observatory, USA Paul Okubo, USGS – Hawaiian Volcano Observatory, USA Ken Hon, University of Hawai'i at Hilo, USA Program Committee Rebecca Carey, University of California, Berkeley, USA Simon Carn, Michigan Technological University, USA Valerie Cayol, Obs. de Physique du Globe de Clermont-Ferrand Helge Gonnermann, Rice University, USA Scott Rowland, SOEST, University of Hawai'i at M noa, USA Financial Support 2 AGU Chapman Conference on Hawaiian Volcanoes: From Source to Surface Site Meeting At A Glance Sunday, 19 August 2012 1600h – 1700h Welcome Reception 1700h – 1800h Introduction and Highlights of Kilauea’s Recent Eruption Activity Monday, 20 August 2012 0830h – 0900h Welcome and Logistics 0900h – 0945h Introduction – Hawaiian Volcano Observatory: Its First 100 Years of Advancing Volcanism 0945h – 1215h Magma Origin and Ascent I 1030h – 1045h Coffee Break 1215h – 1330h Lunch on Your Own 1330h – 1430h Magma Origin and Ascent II 1430h – 1445h Coffee Break 1445h – 1600h Magma Origin and Ascent Breakout Sessions I, II, III, IV, and V 1600h – 1645h Magma Origin and Ascent III 1645h – 1900h Poster Session Tuesday, 21 August 2012 0900h – 1215h Magma Storage and Island Evolution I 1215h – 1330h Lunch on Your Own 1330h – 1445h Magma Storage and Island Evolution II 1445h – 1600h Magma Storage and Island Evolution Breakout Sessions I, II, III, IV, and V 1600h – 1645h Magma Storage -

The Lyman Hawaiian Earthquake Diary, 1833-1917
The Lyman Hawaiian Earthquake Diary, 1833-1917 An amazing accomplishment of record-keeping covering the significant years before, during, and after the strongest earthquake in the history of Hawaii. The 84-year collection of earthquake information credits the perseverance and foresight of Sarah, Isabella, and Frederick Lyman. Recurrent volcanic activity and earthquakes in Hawaii instill among residents respect of the impact of these catastrophic events. The ancient Hawaiians' legend of the journey of the volcano goddess Pele across the Hawaiian Islands, as artistically portrayed by Lisa Koyanagi* of Hila, attests to the persistence of volcanism and earthquakes on the island of Hawaii many centuries ago and concurs with the present scientific understanding of the southeasterly growth of the island chain. *Born and raised on the island of Hawaii, and presently a student of fine arts a the University of Hawaii in Hila. The Lyman Hawaiian Earthquake Diary, 1833-191 7 By MAX WYSS, ROBERT Y. KOYANAGI, and DOAK C. COX U.S. GEOLOGICAL SURVEY BULLETIN 2027 U.S. DEPARTMENT OF THE INTERIOR MANUEL LUJAN, JR., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U. S. Government UNITED STATES GOVERNMENT PRINTING OFFICE: 1992 For sale by Book and Open-File Report Sales U.S. Geological Survey Federal Center, Box 25425 Denver, CO 80225 Library of Congress Cataloging-in-Publication Data Lyman, Sarah Joiner, 1805-1885. The Lyman Hawaiian earthquake diary, 1833-1917 I [edited] by Max Wyss, Robert Y. -

Precontact Vegetation and Soil Nutrient Status in the Shadow of Kohala Volcano, Hawaii ⁎ Oliver A
Geomorphology 89 (2007) 70–83 www.elsevier.com/locate/geomorph Precontact vegetation and soil nutrient status in the shadow of Kohala Volcano, Hawaii ⁎ Oliver A. Chadwick a, , Eugene F. Kelly b, Sara C. Hotchkiss c, Peter M. Vitousek d a Department of Geography, University of California, Santa Barbara, CA 93106, USA b Department of Crop and Soil Science, Colorado State University, Fort Collins, CO 80523, USA c Department of Botany, University of Wisconsin, Madison, WI 53706, USA d Department of Biological Sciences, Stanford University, Stanford, CA 94305, USA Received 13 January 2005; received in revised form 25 July 2006; accepted 25 July 2006 Available online 2 October 2006 Abstract Humans colonized Hawaii about 1200 years ago and have progressively modified vegetation, particularly in mesic to dry 13 tropical forests. We use δ C to evaluate the contribution of C3 and C4 plants to deep soil organic matter to reconstruct pre-human contact vegetation patterns along a wet to dry climate transect on Kohala Mountain, Hawaii Island. Precontact vegetation assemblages fall into three distinct zones: a wet C3 dominated closed canopy forest where annual rainfall is N2000 mm, a dry C4 dominated grassland with annual rainfall b500 mm, and a broad transition zone between these communities characterized by either C3 trees with higher water-use efficiency than the rainforest trees or C3 trees with a small amount of C4 grasses intermixed. The likelihood of C4 grass understory decreases with increasing rainfall. We show that the total concentration of rock-derived nutrients in the b2-mm soil fraction differs in each of these vegetation zones. -

I COPV of HAWAI R SYSTEM FEB 2 3 2018
David LaS5ner UNIVERSITY President 1 I COPV of HAWAI r SYSTEM FEB 2 3 2018 February 12, 2018 ~..., . Mr. Scott Glenn, Director o 0 (X) c::o - ;o Office of Environmental Quality Control l> ...,, r .,., m Department of Health -rri rr, --i :z CD 0 State of Hawai'i -< < ,n 235 South Beretania Street, Room 702 r. :::i -N Oo Honolulu, Hawai'i 96813 :Z :;r.: -i:, < --i7::.,:;=;:-, rn -vi ·"":) Subject: Environmental Impact Statement Preparation Notice for Lancr' := 0 Authorizations for Long-Term Continuation of Astronomy on Maunakea Dear Director Glenn: The University of Hawai'i (UH) has determined at the outset that it will prepare an Environmental Impact Statement (EIS) for its proposed new land authorizations for the continuation of astronomy on Maunakea. UH will prepare the EIS in accordance with the provisions and requirements of Hawai'i Revised Statutes (HRS) Chapter 343. Pursuant to HRS Chapter 343-S(c), an Agency Publication Form and Environmental Impact Statement Preparation Notice (EISPN) are attached. The EISPN includes a description of the requested land authorization and a brief discussion of the kinds of potential environmental impacts which will be analyzed in the forthcoming EIS. In accordance with Hawai'i Administrative Rules Chapter 11-200, we respectfully request that you publish this notice in the next available edition of The Environmental Notice for the public to submit comments to UH during the statutory 30-day public consultation period. If you have any further questions about this letter or its attachments, please contact Stephanie Nagata, Director, Office of Mauna Kea Management, at (808) 933-0734. -

U-Series Isotopic Constraints on Mantle Upwelling on the Periphery of the Hawaiian Plume Isotope Geochemistry of Haleakala Crater Basanites Erin H
U-series isotopic constraints on mantle upwelling on the periphery of the Hawaiian plume Isotope geochemistry of Haleakala crater basanites Erin H. Phillips and Kenneth W.W. Sims, University of Wyoming, and Vincent J.M. Salters, Florida State University 1. Introduction 2. Major and Trace Element Geochemistry and Long-lived Radiogenic Isotopes 100 400 ● The Hawaiian islands are part of the age progressive series of 16 Comparison to Haleakala Crater (this study) 90 global alkaline volcanoes that form the Hawaiian-Emperor seamount chain. At 14 Haleakala SW Rift Zone (Sims et al., 1999) 300 suites Haleakala, on the island of Maui, basanitic lavas of the Hana Haleakala post-shield 80 12 Haleakala shield 200 Volcanics represent end-member rejuvenated stage alkaline 70 Kilauea magmatism (Clauge, 1987). Although shield-stage tholeiitic 100 10 Mauna Loa 60 volcanism predominates in the Hawaiian islands, alkaline lavas O 2 Mauna Kea 0 erupted on the trailing edge of the Hawaiian plume present an 8 Hualalai trachytes 50 O+Na important component to understanding mantle melting and solid 2 Loihi K 40 6 Nyiragongo mantle upwelling. Averages for 30 Leucite Hills lamproites Hawaiian Lavas 4 Ross Island, Antarctica basanites 20 ● We present geochemical data for 13 samples from Haleakala Samoa crater. 14C ages for seven samples range from 870 ± 40 to 4070 ± 50 2 See supplement for full reference list 10 years (Sherrod and McGeehin, 1999). Preliminary data for 5 0 1995) Mantle (McDonough and Sun, Sample/Primitive 0 samples from the Haleakala southwest rift zone (Sims et al., 1999) 35 40 45 50 55 60 65 Rb Ba Th U Nb La Ce Sr Pb Nd Sm Hf Eu Dy Y Yb Lu SiO are consistent with iso-viscous (Watson and McKenzie, 1991) and 2 thermo-viscous (Hauri et al., 1994) fluid mechanical models of 10 plume upwelling in which upwelling rates are slower on the Hawaiian Lavas periphery of plume. -

Madam Pele: Novel and Essay
Edith Cowan University Research Online Theses: Doctorates and Masters Theses 2006 Madam Pele: Novel and essay Jud L. House Edith Cowan University Follow this and additional works at: https://ro.ecu.edu.au/theses Part of the Creative Writing Commons Recommended Citation House, J. L. (2006). Madam Pele: Novel and essay. https://ro.ecu.edu.au/theses/37 This Thesis is posted at Research Online. https://ro.ecu.edu.au/theses/37 Edith Cowan University Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorize you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. Where the reproduction of such material is done without attribution of authorship, with false attribution of authorship or the authorship is treated in a derogatory manner, this may be a breach of the author’s moral rights contained in Part IX of the Copyright Act 1968 (Cth). Courts have the power to impose a wide range of civil and criminal sanctions for infringement of copyright, infringement of moral rights and other offences under the Copyright Act 1968 (Cth). Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. USE OF THESIS The Use of Thesis statement is not included in this version of the thesis. -

Pele Is Angry, but Oh So Beautiful
CANADIAN FLIGHT A Hundred Things by Bryan Quickmire Pele Is Angry, But Oh So Beautiful The next day we depart on our positioning flight from A long time ago, in a land far away, the Honolulu to Kauai, Pele’s first Hawaiian residence. The goddesses were battling. Pele, goddess of fire, hundred miles is mostly over deep blue Pacific, mostly out of sight of land. In the event of the untimely demise was defeated by Namaka, goddess of the sea. of the engine we wear inflatable life vests at all times and Banished, Pele set sail in a sacred canoe, from carry a life raft and survival equipment. Kahiki out across the empty Pacific. Upon making landfall, we commence a clockwise tour. The pinnacle we are circling, Mount Waialeale, is the Three thousand miles later she came upon the Hawaiian wettest place on earth, enjoying 460 inches of rain each Islands and settled on the island of Kauai. There she year. If that amount of moisture fell as snow it would be lived until one day the vengeful Namaka found her and literally a mile high! The runoff from this continuous put out her fires with salt water and tidal waves. deluge has created the Waimea Canyon, known as the Pele moved to Oahu, then Molokai, then Maui, each time Grand Canyon of the Pacific. We explore this 3,000 foot forced onward by the wrath of her more powerful sister. deep gorge, ever mindful of the unyielding walls and the Finally, after the fiercest battle of all, she went to the Big floor which rises into the cap clouds.