Magmas Parental to the Chassigny Meteorite: New Considerations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Linking the Chassigny Meteorite and the Martian Surface Rock Backstay: Insights Into Igneous Crustal Differentiation Processes on Mars
Meteoritics & Planetary Science 44, Nr 6, 853–869 (2009) Abstract available online at http://meteoritics.org Linking the Chassigny meteorite and the Martian surface rock Backstay: Insights into igneous crustal differentiation processes on Mars Hanna NEKVASIL*, Francis M. MCCUBBIN, Andrea HARRINGTON, Stephen ELARDO, and Donald H. LINDSLEY Department of Geosciences, Stony Brook University, Stony Brook, New York 11794–2100, USA *Corresponding author. E-mail: [email protected] (Received 05 August 2008; revision accepted 18 March 2009) Abstract–In order to use igneous surface lithologies to constrain Martian mantle characteristics, secondary processes that lead to compositional modification of primary mantle melts must be considered. Crystal fractionation of a mantle-derived magma at the base of the crust followed by separation and ascent of residual liquids to the surface is common in continental hotspot regions on Earth. The possibility that this process also takes place on Mars was investigated by experimentally determining whether a surface rock, specifically the hawaiite Backstay analyzed by the MER Spirit could produce a known cumulate lithology with a deep origin (namely the assemblages of the Chassigny meteorite) if trapped at the base of the Martian crust. Both the major cumulus and melt inclusion mineral assemblages of the Chassigny meteorite were produced experimentally by a liquid of Backstay composition within the pressure range 9.3 to 6.8 kbar with bulk water contents between 1.5 and 2.6 wt%. Experiments at 4.3 and 2.8 kbar did not produce the requisite assemblages. This agreement suggests that just as on Earth, Martian mantle-derived melts may rise to the surface or remain trapped at the base of the crust, fractionate, and lose their residual liquids. -

Source to Surface Model of Monogenetic Volcanism: a Critical Review
Downloaded from http://sp.lyellcollection.org/ by guest on September 28, 2021 Source to surface model of monogenetic volcanism: a critical review I. E. M. SMITH1 &K.NE´ METH2* 1School of Environment, University of Auckland, Auckland, New Zealand 2Volcanic Risk Solutions, Massey University, Palmerston North 4442, New Zealand *Correspondence: [email protected] Abstract: Small-scale volcanic systems are the most widespread type of volcanism on Earth and occur in all of the main tectonic settings. Most commonly, these systems erupt basaltic magmas within a wide compositional range from strongly silica undersaturated to saturated and oversatu- rated; less commonly, the spectrum includes more siliceous compositions. Small-scale volcanic systems are commonly monogenetic in the sense that they are represented at the Earth’s surface by fields of small volcanoes, each the product of a temporally restricted eruption of a composition- ally distinct batch of magma, and this is in contrast to polygenetic systems characterized by rela- tively large edifices built by multiple eruptions over longer periods of time involving magmas with diverse origins. Eruption styles of small-scale volcanoes range from pyroclastic to effusive, and are strongly controlled by the relative influence of the characteristics of the magmatic system and the surface environment. Gold Open Access: This article is published under the terms of the CC-BY 3.0 license. Small-scale basaltic magmatic systems characteris- hazards associated with eruptions, and this is tically occur at the Earth’s surface as fields of small particularly true where volcanic fields are in close monogenetic volcanoes. These volcanoes are the proximity to population centres. -

31 a Preliminary Study Of
31 A PRELIMINARY STUDY OF THE: TERTIARY VOLCANIC AND SEDIMENTARY ROCKS, GÜMELE, ESKİŞEHİR Eskişehir, Gümele Çevresindeki Tersiyer Volkanik ve Sedimanter Kayaçlarda Bir Ön Çalışma Taylan Lünel Middle East Technical University, Department of Geological Engineering Ankara ÖZ. — Seyitgazi-Eskişehir antiklinoriumu'nun çok fazla deforme olmuş ve metamorfizmaya uğramış kayaçlarının kuzey-kuzeybatısında bulunan sedimanter ve volkanik kayaçlar incelenmiştir. Karasal ve gölsel fasiyesde meydana gelen Tersi- yer sedimanter kayaçlar Güney Eskişehir küvetinde olunmuşlardır. Karasal fasiye- si meydana getiren kayaç birimlerini kaba kumtaşları, kumtaşları, bitki kalıntıları ihtiva eden kil ve marnlar ve serpantinit blokları taşıyan bazal konglomerası teşkil etmektedir. Gölsel fasiyes ise genellikle killi ve tüflü kalkerler, kalkerler, marnlar, kon- glomeralar ve tüflerden meydana gelmiştir. Küvetteki en eski sedimanlar ve piroklas- tikler Alt Miosen'de oluşmuşlardır. Yataya yakın konumlanmış bazik-intermediyar lav akıntıları Pliosen yaşlı olup Altüst Neojen sedimantasyon kesikliğinde meyda- na gelmiştir. Üst Neojen sedimanter kayaçları intermediyar-basaltik volkaniklerin üzerinde ince bandlar şeklinde bulunurlar. Bu birim marn ve kalkerlerden meyda- na gelmiştir. Alt Miosen’de asid volkanik faaliyetler neticesinde meydana gelen sil- lar (unweldd tuffs) oligomikt konglomeralardan evvel teşekkül etmiştir. Bu volkanik aktitivite muhtemelen kesikli ve kısıtlı olarak devam etmiş ve tüflü kalkerleri mey- dana getirmiştir. Pliosen yaşlı bazik-intermediyar -
![Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M](https://docslib.b-cdn.net/cover/9633/wednesday-march-22-2017-w453-martian-meteorite-madness-mixing-on-a-variety-of-scales-1-30-p-m-489633.webp)
Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M
Lunar and Planetary Science XLVIII (2017) sess453.pdf Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING ON A VARIETY OF SCALES 1:30 p.m. Waterway Ballroom 5 Chairs: Arya Udry Geoffrey Howarth 1:30 p.m. Nielsen S. G. * Magna T. Mezger K. The Vanadium Isotopic Composition of Mars and Evidence for Solar System Heterogeneity During Planetary Accretion [#1225] Vanadium isotope composition of Mars distinct from Earth and chondrites. 1:45 p.m. Tait K. T. * Day J. M. D. Highly Siderophile Element and Os-Sr Isotope Systematics of Shergotittes [#3025] The shergottite meteorites represent geochemically diverse, broadly basaltic, and magmatically-derived rocks from Mars. New samples were processed and analyzed. 2:00 p.m. Armytage R. M. G. * Debaille V. Brandon A. D. Agee C. B. The Neodymium and Hafnium Isotopic Composition of NWA 7034, and Constraints on the Enriched End-Member for Shergottites [#1065] Couple Sm-Nd and Lu-Hf isotopic systematics in NWA 7034 suggest that such a crust is not the enriched end-member for shergottites. 2:15 p.m. Howarth G. H. * Udry A. Nickel in Olivine and Constraining Mantle Reservoirs for Shergottite Meteorites [#1375] Ni enrichment in olivine from enriched versus depleted shergottites provide evidence for constraining mantle reservoirs on Mars. 2:30 p.m. Jean M. M. * Taylor L. A. Exploring Martian Mantle Heterogeneity: Multiple SNC Reservoirs Revealed [#1666] The objective of the present study is to assess how many mixing components can be recognized, and address ongoing debates within the martian isotope community. 2:45 p.m. Udry A. * Day J. -

THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution
VOLCANISM IN HAWAII Chapter 1 - .-............,. THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution By David A. Clague and G. Brent Dalrymple ABSTRACT chain, the near-fixity of the hot spot, the chemistry and timing of The Hawaiian-Emperor volcanic chain stretches nearly the eruptions from individual volcanoes, and the detailed geom 6,000 km across the North Pacific Ocean and consists of at least etry of volcanism. None of the geophysical hypotheses pro t 07 individual volcanoes with a total volume of about 1 million posed to date are fully satisfactory. However, the existence of km3• The chain is age progressive with still-active volcanoes at the Hawaiian ewell suggests that hot spots are indeed hot. In the southeast end and 80-75-Ma volcanoes at the northwest addition, both geophysical and geochemical hypotheses suggest end. The bend between the Hawaiian and .Emperor Chains that primitive undegassed mantle material ascends beneath reflects a major change in Pacific plate motion at 43.1 ± 1.4 Ma Hawaii. Petrologic models suggest that this primitive material and probably was caused by collision of the Indian subcontinent reacts with the ocean lithosphere to produce the compositional into Eurasia and the resulting reorganization of oceanic spread range of Hawaiian lava. ing centers and initiation of subduction zones in the western Pacific. The volcanoes of the chain were erupted onto the floor of the Pacific Ocean without regard for the age or preexisting INTRODUCTION structure of the ocean crust. Hawaiian volcanoes erupt lava of distinct chemical com The Hawaiian Islands; the seamounts, hanks, and islands of positions during four major stages in their evolution and the Hawaiian Ridge; and the chain of Emperor Seamounts form an growth. -
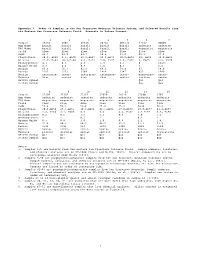
1 Appendix 3. Modes of Samples in the San Francisco Mountain
Appendix 3. Modes of Samples in the San Francisco Mountain Volcanic System, and Selected Basalts from the Eastern San Francisco Volcanic Field. Minerals in Volume Percent. 1 2 3 4 5 6 7 Sample 3823A 2A06 2812A 2031A 2031.B 3732J DC04B Map Name basalt basalt basalt basalt basalt andesite andesite TAS Name basalt basalt basalt basalt basalt mugearite mugearite Field flow flow flow flow flow flow flow SiO2 48.7 48.9 49.7 50.4 51.0 52.2 53.0 Plagioclase 14.6, An69 8.3, An70 25.6,An67 25.5,An71 20.2,An74 29, An64 32.0,An62 Olivine 11.2, Fo83 10.9,Fo84 8.1, Fo73 7.0, Fo77 3.9, Fo76 3, Fo75 6.6, Fo75 Clinopyroxene 4.3 4.3 4.6 1.8 1.3 0 trace Opaque Oxide 4.1 0.2 0.5 1.6 0.3 1 2.4 Matrix 65.8 76.3 61.2 64.1 74.4 67 59.0 Points 1165 1173 1504 1464 1347 827 1549 Matrix intergran- inter- intergran- intergran- inter- microcrys- inter- Texture ular sertal ular ular sertal talline sertal MF1959 Symbol Qa1 Qa1 I-1663 Symbol Qao Qao 8 9 10 11 12 13 14 Sample 3732K 3732Z 3732Y 2705D 3831A 3729Q 3707 Map Name andesite andesite andesite andesite andesite andesite andesite TAS Name mugearite mugearite mugearite mugearite mugearite mugearite mugearite Field flow flow dike flow flow flow flow SiO2 53.4 53.7 53.8 55.2 55.3 56.0 56.6 Plagioclase 24.8,An64 15.3,An61 30.2,An61 42.1,An52 27.0,An62 29.8,An57 41.2,An57 Olivine 2.8, Fo69 3.1, Fo65 4.1 5.6 4.3, Fo62 3.7 Fo57 2.5, Fo58 Clinopyroxene 0.2 0.5 1.5 2.1 0.8 3.0 1.1 Orthopyroxene - - 0.5 - - 0.2 - Opaque Oxide 0.3 0.8 3.0 1.0 0.6 2.1 1.4 Matrix 71.8 80.3 60.7 49.2 67.2 61.1 53.8 Points 1196 1785 854 1844 1428 1029 1540 Matrix hyalo- inter- inter- hyalo- hyalo- hyalo- micro- Texture pilitic granular sertal ophitic pilitic ophitic crystalline MF1959 Symbol Qa1 Qa1 Qai Qa1 Qa2 Qa2 Qa2 I-1663 Symbol Qao Qao Qai Qao Qay Qay Qay Notes: 1. -

Direct Derivation of Benmoreite to Phonolite
EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES Didier LAPORTE, Sarah LAMBART, Pierre SCHIANO, and Luisa OTTOLINI SUPPLEMENTARY MATERIAL 1. Experimental and analytical techniques 1.1 Starting materials. The starting material used to prepare our two fertile mantle compositions was a fresh spinel lherzolite xenolith (Bri3) from Mont Briançon volcano, French Massif Central. Because a fine grain size is critical to attain chemical equilibrium, lherzolite Bri3 was first pulverized in a micronizing mill for 30 min to reduce its grain size to 2-4 µm. The powder was then fired 5 hours at 900°C in a CO2/H2 atmosphere with gas flow rates adjusted to yield an oxygen fugacity between the magnetite-wüstite and the iron-wüstite -15.91 buffers (fO2 = 10 bar). As Bri3 contains only 100 ppm K2O, small amounts of synthetic basalt B2 were added to Bri3 to prepare the fertile mantle compositions MBK (410 ppm K2O) and MBK+ (930 ppm K2O; compositions Bri3, B2, MBK and MBK+ are given in Table 1). Mixtures of Bri3 and B2 were homogenized by grinding in an agate mortar for one hour. The starting material for reversal experiments MBK+13 and MBK+14 was a mixture of 80.16 % MBK+ and 19.84 % phonolite gel +6 (Table 1). Phonolite gel +6 was prepared using the gel method (Luth and Ingamells, 1965), and then fired at the same T and fO2 as lherzolite Bri3. Gel +6 matches well the target composition (that is, the glass in partial melting experiment MBK+6), except that it is ≈ 10 % richer in Na2O. -

Geologic Map of the State of Hawai 'I
Geologic Map of the State of Hawai‘i By David R. Sherrod, John M. Sinton, Sarah E. Watkins, and Kelly M. Brunt Open-File Report 2007–1089 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested citation: Sherrod, D.R., Sinton, J.M., Watkins, S.E., and Brunt, K.M., 2007, Geologic Map of the State of Hawai`i: U.S. Geological Survey Open-File Report 2007-1089, 83 p., 8 plates, scales 1:100,000 and 1:250,000, with GIS database Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. ii Geologic Map of the State of Hawai‘i By David R. Sherrod, John M. Sinton, Sarah E. Watkins, and Kelly M. Brunt About this map Sources of mapping, methods of This geologic map and its digital databases present compilation, origin of stratigraphic the geology of the eight major islands of the State of names, and divisions of the geologic Hawai‘i. -

The Nakhlite Meteorites: Augite-Rich Igneous Rocks from Mars ARTICLE
ARTICLE IN PRESS Chemie der Erde 65 (2005) 203–270 www.elsevier.de/chemer INVITED REVIEW The nakhlite meteorites: Augite-rich igneous rocks from Mars Allan H. Treiman Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058-1113, USA Received 22 October 2004; accepted 18 January 2005 Abstract The seven nakhlite meteorites are augite-rich igneous rocks that formed in flows or shallow intrusions of basaltic magma on Mars. They consist of euhedral to subhedral crystals of augite and olivine (to 1 cm long) in fine-grained mesostases. The augite crystals have homogeneous cores of Mg0 ¼ 63% and rims that are normally zoned to iron enrichment. The core–rim zoning is cut by iron-enriched zones along fractures and is replaced locally by ferroan low-Ca pyroxene. The core compositions of the olivines vary inversely with the steepness of their rim zoning – sharp rim zoning goes with the most magnesian cores (Mg0 ¼ 42%), homogeneous olivines are the most ferroan. The olivine and augite crystals contain multiphase inclusions representing trapped magma. Among the olivine and augite crystals is mesostasis, composed principally of plagioclase and/or glass, with euhedra of titanomagnetite and many minor minerals. Olivine and mesostasis glass are partially replaced by veinlets and patches of iddingsite, a mixture of smectite clays, iron oxy-hydroxides and carbonate minerals. In the mesostasis are rare patches of a salt alteration assemblage: halite, siderite, and anhydrite/ gypsum. The nakhlites are little shocked, but have been affected chemically and biologically by their residence on Earth. Differences among the chemical compositions of the nakhlites can be ascribed mostly to different proportions of augite, olivine, and mesostasis. -

The Tissint Martian Meteorite As Evidence for the Largest Impact Excavation
ARTICLE Received 17 Jul 2012 | Accepted 20 Dec 2012 | Published 29 Jan 2013 DOI: 10.1038/ncomms2414 The Tissint Martian meteorite as evidence for the largest impact excavation Ioannis P. Baziotis1, Yang Liu1,2, Paul S. DeCarli3, H. Jay Melosh4, Harry Y. McSween1, Robert J. Bodnar5 & Lawrence A. Taylor1 High-pressure minerals in meteorites provide clues for the impact processes that excavated, launched and delivered these samples to Earth. Most Martian meteorites are suggested to have been excavated from 3 to 7 km diameter impact craters. Here we show that the Tissint meteorite, a 2011 meteorite fall, contains virtually all the high-pressure phases (seven minerals and two mineral glasses) that have been reported in isolated occurrences in other Martian meteorites. Particularly, one ringwoodite (75 Â 140 mm2) represents the largest grain observed in all Martian samples. Collectively, the ubiquitous high-pressure minerals of unusually large sizes in Tissint indicate that shock metamorphism was widely dispersed in this sample (B25 GPa and B2,000 1C). Using the size and growth kinetics of the ring- woodite grains, we infer an initial impact crater with B90 km diameter, with a factor of 2 uncertainty. These energetic conditions imply alteration of any possible low-T minerals in Tissint. 1 Planetary Geosciences Institute, Department of Earth and Planetary Sciences, University of Tennessee, Knoxville, Tennessee 37996, USA. 2 Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109, USA. 3 Poulter Laboratory, SRI International, Menlo Park, California 94025, USA. 4 Department of Earth, Atmospheric and Planetary Sciences, Purdue University, West Lafayette, Indiana 47907, USA. 5 Department of Geosciences, Virginia Tech, Blacksburg, Virginia 24061, USA. -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

Origin and Emplacement of a Composite Benmoreite-Trachyte
. I Origin and emplacement of a composite benmoreite - trachyte intrusion: Castle Rock, Lytt~lton Volcano A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Geology at the University of Canterbury by Rose Fitzgerald University of Canterbury 1991 II " .. a remarkable dyke forms the crest of the hill, the almost vertical sides of which are in marked contrast with the rounded slopes immediately below it." (Dobson, 1880). I I I Abstract Castle Rock and the Eastern Sheet are elongate intrusions associated with the Lyttelton Volcano, Banks Peninsula. These intrusions were emplaced about 9.7 Ma, late in the evolutionary history of the volcano, and are connected to feeder dikes of the Lyttelton radial swarm. Trachytes, Groups A and B, and benmoreites, low silica and high silica, are components of Castle Rock, whereas only Group A trachyte is found within the Eastern Sheet. The flow foliation and cooling joints of the trachytes and benmoreites indicate the emplacement of bodies with domal cross - sectional shape in a single episode. Velocity gradients in the magmas during emplacement produced groundmass crenulations subsequent to the development of a strong preferred orientation. These crenulations enabled the growing· domes to continuously change their shapes, thus facilitating magma movement within. During the emplacement of Castle Rock magma flow was generally away from the magma source, however, restricted magma movement at the northern end during the trachyte intrusion produced magma movement back towards its origin. Overriding of the trachyte and benmoreite magmas at the advancing magma front did not occur during the emplacement of Castle Rock.