Linking the Chassigny Meteorite and the Martian Surface Rock Backstay: Insights Into Igneous Crustal Differentiation Processes on Mars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M](https://docslib.b-cdn.net/cover/9633/wednesday-march-22-2017-w453-martian-meteorite-madness-mixing-on-a-variety-of-scales-1-30-p-m-489633.webp)
Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M
Lunar and Planetary Science XLVIII (2017) sess453.pdf Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING ON A VARIETY OF SCALES 1:30 p.m. Waterway Ballroom 5 Chairs: Arya Udry Geoffrey Howarth 1:30 p.m. Nielsen S. G. * Magna T. Mezger K. The Vanadium Isotopic Composition of Mars and Evidence for Solar System Heterogeneity During Planetary Accretion [#1225] Vanadium isotope composition of Mars distinct from Earth and chondrites. 1:45 p.m. Tait K. T. * Day J. M. D. Highly Siderophile Element and Os-Sr Isotope Systematics of Shergotittes [#3025] The shergottite meteorites represent geochemically diverse, broadly basaltic, and magmatically-derived rocks from Mars. New samples were processed and analyzed. 2:00 p.m. Armytage R. M. G. * Debaille V. Brandon A. D. Agee C. B. The Neodymium and Hafnium Isotopic Composition of NWA 7034, and Constraints on the Enriched End-Member for Shergottites [#1065] Couple Sm-Nd and Lu-Hf isotopic systematics in NWA 7034 suggest that such a crust is not the enriched end-member for shergottites. 2:15 p.m. Howarth G. H. * Udry A. Nickel in Olivine and Constraining Mantle Reservoirs for Shergottite Meteorites [#1375] Ni enrichment in olivine from enriched versus depleted shergottites provide evidence for constraining mantle reservoirs on Mars. 2:30 p.m. Jean M. M. * Taylor L. A. Exploring Martian Mantle Heterogeneity: Multiple SNC Reservoirs Revealed [#1666] The objective of the present study is to assess how many mixing components can be recognized, and address ongoing debates within the martian isotope community. 2:45 p.m. Udry A. * Day J. -

The Nakhlite Meteorites: Augite-Rich Igneous Rocks from Mars ARTICLE
ARTICLE IN PRESS Chemie der Erde 65 (2005) 203–270 www.elsevier.de/chemer INVITED REVIEW The nakhlite meteorites: Augite-rich igneous rocks from Mars Allan H. Treiman Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058-1113, USA Received 22 October 2004; accepted 18 January 2005 Abstract The seven nakhlite meteorites are augite-rich igneous rocks that formed in flows or shallow intrusions of basaltic magma on Mars. They consist of euhedral to subhedral crystals of augite and olivine (to 1 cm long) in fine-grained mesostases. The augite crystals have homogeneous cores of Mg0 ¼ 63% and rims that are normally zoned to iron enrichment. The core–rim zoning is cut by iron-enriched zones along fractures and is replaced locally by ferroan low-Ca pyroxene. The core compositions of the olivines vary inversely with the steepness of their rim zoning – sharp rim zoning goes with the most magnesian cores (Mg0 ¼ 42%), homogeneous olivines are the most ferroan. The olivine and augite crystals contain multiphase inclusions representing trapped magma. Among the olivine and augite crystals is mesostasis, composed principally of plagioclase and/or glass, with euhedra of titanomagnetite and many minor minerals. Olivine and mesostasis glass are partially replaced by veinlets and patches of iddingsite, a mixture of smectite clays, iron oxy-hydroxides and carbonate minerals. In the mesostasis are rare patches of a salt alteration assemblage: halite, siderite, and anhydrite/ gypsum. The nakhlites are little shocked, but have been affected chemically and biologically by their residence on Earth. Differences among the chemical compositions of the nakhlites can be ascribed mostly to different proportions of augite, olivine, and mesostasis. -

The Tissint Martian Meteorite As Evidence for the Largest Impact Excavation
ARTICLE Received 17 Jul 2012 | Accepted 20 Dec 2012 | Published 29 Jan 2013 DOI: 10.1038/ncomms2414 The Tissint Martian meteorite as evidence for the largest impact excavation Ioannis P. Baziotis1, Yang Liu1,2, Paul S. DeCarli3, H. Jay Melosh4, Harry Y. McSween1, Robert J. Bodnar5 & Lawrence A. Taylor1 High-pressure minerals in meteorites provide clues for the impact processes that excavated, launched and delivered these samples to Earth. Most Martian meteorites are suggested to have been excavated from 3 to 7 km diameter impact craters. Here we show that the Tissint meteorite, a 2011 meteorite fall, contains virtually all the high-pressure phases (seven minerals and two mineral glasses) that have been reported in isolated occurrences in other Martian meteorites. Particularly, one ringwoodite (75 Â 140 mm2) represents the largest grain observed in all Martian samples. Collectively, the ubiquitous high-pressure minerals of unusually large sizes in Tissint indicate that shock metamorphism was widely dispersed in this sample (B25 GPa and B2,000 1C). Using the size and growth kinetics of the ring- woodite grains, we infer an initial impact crater with B90 km diameter, with a factor of 2 uncertainty. These energetic conditions imply alteration of any possible low-T minerals in Tissint. 1 Planetary Geosciences Institute, Department of Earth and Planetary Sciences, University of Tennessee, Knoxville, Tennessee 37996, USA. 2 Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109, USA. 3 Poulter Laboratory, SRI International, Menlo Park, California 94025, USA. 4 Department of Earth, Atmospheric and Planetary Sciences, Purdue University, West Lafayette, Indiana 47907, USA. 5 Department of Geosciences, Virginia Tech, Blacksburg, Virginia 24061, USA. -
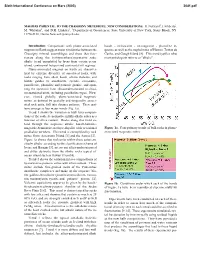
Magmas Parental to the Chassigny Meteorite: New Considerations
Sixth International Conference on Mars (2003) 3041.pdf MAGMAS PARENTAL TO THE CHASSIGNY METEORITE: NEW CONSIDERATIONS. H. Nekvasil1, J. Filiberto1, M. Whitaker1, and D.H. Lindsley1, 1Department of Geosciences, State University of New York, Stony Brook, NY 11794-2100, [email protected]. Introduction: Comparisons with plume-associated basalt - ne-hawaiite - ne-mugearite - phonolite se- magmas on Earth suggest major similarities between the quence as well as the nephelinites of Hawaii, Tristan da Chassigny mineral assemblages and those that frac- Cunha, and Gough Island [8]. This trend typifies what tionate along the hy(hypersthene)-normative sodic most petrologists refer to as "alkalic". alkalic trend exemplified by lavas from certain ocean island, continental hotspot and continental rift regimes. Plume-associated magmas on Earth are character- ized by extreme diversity of associated rocks, with rocks ranging from alkali basalt, olivine tholeiite, and biotite gabbro to anorthosite, syenite, comendite, pantellerite, phonolite and potassic granite, and span- ning the spectrum from silica-undersaturated to silica- oversaturated units, including peralkaline types. How- ever, viewed globally, plume-associated magmatic suites, as defined by spatially and temporally associ- ated rock units, fall into distinct patterns. These pat- terns emerge as four major trends (Fig. 1a). Trend 1 shows the variation in bulk lava composi- tions of the sodic hy-normative mildly alkalic suites as a function of silica content. Rocks along this trend ex- tend through the sequence alkalic basalt-hawaiite- mugearite-benmoreite-trachyte-rhyolite with occasional Figure 1a. Four primary trends of bulk rocks in plume- peralkaline members. This trend is exemplified by rock associated magmatic suites. -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

Spectrum Winter.Pmd
SPECTRUM Winter 2006, Vol. 31, No. 3 The Journal of the Illinois Science Teachers Association In this Issue: Meteorites and Moon Rocks Physical Science Teaching Strategies ista Literacy Infusion Plan Ahead: ISTA Conference - November 3-4, 2006 Science in the South - March 10, 2006 EEAI Conference - May 4-6, 2006 Illinois Science Teachers Spectrum Association The Journal of the Illinois Science Teachers Association Volume 31, Number 3 Executive Committee Raymond J. Dagenais President Illinois Mathematics and Science Academy Spectrum is published three times per year, in spring, fall, and 1500 W. Sulllivan Road winter, by the Illinois Science Teachers Association, Illinois Math- Aurora, IL 60506 ematics and Science Academy, 1500 W. Sullivan Rd., Aurora, IL [email protected] 60506. Subscription rates are found with the membership informa- tion. Subscription inquiries should be directed to Sherry Duncan Marylin Lisowski (email: [email protected]). Past-President Eastern Illinois University Send submissions and inquiries to the editor. Articles should be 600 Lincoln Avenue directed to individual area focus editors (see next page and write Charleston, IL 61920 for the SPECTRUM information). [email protected] Judith A. Scheppler, Ph.D. Denny Moore Coordinator of Student Inquiry Vice President Director of the Grainger Center for Imagination and Inquiry John Deere Middle School Illinois Mathematics and Science Academy 2035 11th St. 1500 West Sullivan Road Moline, IL 61265 Aurora, IL 60506 [email protected] [email protected] Jill Carter On the cover: When is it Time for Science? (See article on page President-Elect 14.) Photographs courtesy of the Center@IMSA. Pekin Community H.S. 320 Stadium Dr. -

Extra-Terrestrial Igneous Granites and Related Rocks: a Review of Their Occurrence and Petrogenesis
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Lithos 153 (2012) 3–24 Contents lists available at SciVerse ScienceDirect Lithos journal homepage: www.elsevier.com/locate/lithos Extra-terrestrial igneous granites and related rocks: A review of their occurrence and petrogenesis Bernard Bonin ⁎ UMR8148 ‘IDES’, CNRS, Département des Sciences de la Terre, Université de Paris-Sud, F-91405 ORSAY CEDEX, France article info abstract Article history: The telluric planets and the asteroid belt display the same internal structure with a metallic inner core and a Received 4 November 2011 silicate outer shell. Experimental data and petrological evidence in silicate systems show that granite can be Accepted 4 April 2012 produced by extreme igneous differentiation through various types of igneous processes. Available online 14 April 2012 On Moon, 4.4–3.9 Ga granite clasts display dry mineral assemblages. They correspond to at least 8 discrete intru- sive events. Large K/Ca enrichment and low REE abundances in granite relative to KREEP are consistent with sil- Keywords: icate liquid immiscibility, a process observed in melt inclusions within olivine of lunar basalts and in lunar Planetary granites fi A-type meteorites. -

List G - Meteorites - Alphabetical List
LIST G - METEORITES - ALPHABETICAL LIST Specific name Group name Specific name Group name Abee Meteorite EH chondrites EETA 79001 Elephant Moraine Meteorites Acapulco Meteorite acapulcoite and shergottite acapulcoite stony meteorites Efremovka Meteorite CV chondrites Acfer Meteorites meteorites EH chondrites enstatite chondrites achondrites stony meteorites EL chondrites enstatite chondrites ALH 84001 Allan Hills Meteorites and Elephant Moraine meteorites achondrites Meteorites ALHA 77005 Allan Hills Meteorites and enstatite chondrites chondrites shergottite eucrite achondrites ALHA 77307 Allan Hills Meteorites and Fayetteville Meteorite H chondrites CO chondrites Frontier Mountain meteorites ALHA 81005 Allan Hills Meteorites and Meteorites achondrites Gibeon Meteorite octahedrite Allan Hills Meteorites meteorites GRA 95209 Graves Nunataks Meteorites Allende Meteorite CV chondrites and lodranite angrite achondrites Graves Nunataks meteorites Ashmore Meteorite H chondrites Meteorites Asuka Meteorites meteorites H chondrites ordinary chondrites ataxite iron meteorites Hammadah al Hamra meteorites aubrite achondrites Meteorites Barwell Meteorite L chondrites Haveroe Meteorite ureilite Baszkowka Meteorite L chondrites Haviland Meteorite H chondrites Belgica Meteorites meteorites HED meteorites achondrites Bencubbin Meteorite chondrites Hedjaz Meteorite L chondrites Bishunpur Meteorite LL chondrites Henbury Meteorite octahedrite Bjurbole Meteorite L chondrites hexahedrite iron meteorites Brenham Meteorite pallasite HL chondrites ordinary chondrites -

References for ALH84001 (Compiled by C
References for ALH84001 (compiled by C. Meyer, Oct 2012) Akai J. (1997) Characteristics of iron-oxide and iron-sulfide grains in meteorites and terrestrial sediments, with special references to magnetite grains in Allan Hills 84001 (abs). Meteorit. & Planet. Sci. 32, A5. Anders Edward (1996) Evaluating the evidence for past life on Mars. Science 274, 2119-2120. Antretter M. and Fuller M. (2001) Paleomagnetic and rock magnetic studies of ALH84001 (abs). Meteorit. & Planet. Sci. 36, A11-12. Antretter M., Fuller M., Scott E., Jackson M., Moskowitz B. and Solheid P. (2003) Paleomagnetic record of Martian meteorite ALH84001. J. of Geophys. Res. 108, 5049, doi:10.1029/202JE001979 Ash R.D., Knott S.F. and Turner Grenvile (1995) Evidence for the timing of the early bombardment of Mars (abs). Meteoritics 30, 483. Ash R.D., Knott S.F. and Turner G. (1996) A 4-Gyr shock age for a Martian meteorite and implications for the cratering history of Mars. Nature 380, 57-59. Bada J.L., Glavin D.P., McDonald G.D. and Becker L. (1998a) Amino acids in the ALH84001 Martian meteorite (abs#1894). Lunar Planet. Sci. XXIX Lunar Planetary Institute, Houston. Bada J.L., Glavin D.P., McDonald G.D. and Becker L. (1998b) A search for endogenous amino acids in Martian meteorite ALH84001. Science 279, 362-365. Baker L., Franchi I.A., Wright I.P. and Pillinger C.T. (1998) Oxygen isotopes in water from Martian meteorites (abs). Meteorit. & Planet. Sci. 33, A11-12. Barber D.J., Scott E.R.D. and Consolmagno G. (2001) Transmission electron microscopy of carbonates and associated minerals in ALH84001: Impact-induced deformation and carbonate decomposition (abs). -

Ejection of Martian Meteorites
Meteoritics & Planetary Science 40, Nr 9/10, 1393–1411 (2005) Abstract available online at http://meteoritics.org Ejection of Martian meteorites Jˆrg FRITZ1*, Natalia ARTEMIEVA2, and Ansgar GRESHAKE1 1Institut f¸r Mineralogie, Museum f¸r Naturkunde, Humboldt-Universit‰t zu Berlin, Invalidenstrasse 43, 10115 Berlin, Germany 2Institute for Dynamics of Geospheres, Russian Academy of Science, Leninsky Prospect 38, Building 1, Moscow 119334, Russia *Corresponding author. E-mail: [email protected] (Received 29 March 2005; revision accepted 11 April 2005) Abstract–We investigated the transfer of meteorites from Mars to Earth with a combined mineralogical and numerical approach. We used quantitative shock pressure barometry and thermodynamic calculations of post-shock temperatures to constrain the pressure/temperature conditions for the ejection of Martian meteorites. The results show that shock pressures allowing the ejection of Martian meteorites range from 5 to 55 GPa, with corresponding post-shock temperature elevations of 10 to about 1000 °C. With respect to shock pressures and post-shock temperatures, an ejection of potentially viable organisms in Martian surface rocks seems possible. A calculation of the cooling time in space for the most highly shocked Martian meteorite Allan Hills (ALH) 77005 was performed and yielded a best-fit for a post-shock temperature of 1000 °C and a meteoroid size of 0.4 to 0.6 m. The final burial depths of the sub-volcanic to volcanic Martian rocks as indicated by textures and mineral compositions of meteorites are in good agreement with the postulated size of the potential source region for Martian meteorites during the impact of a small projectile (200 m), as defined by numerical modeling (Artemieva and Ivanov 2004). -

MARTIAN METEORITES 5:30 P.M
80th Annual Meeting of the Meteoritical Society 2017 (LPI Contrib. No. 1987) sess604.pdf Thursday, July 27, 2017 POSTER SESSION II: MARTIAN METEORITES 5:30 p.m. Poster Area McCubbin F. M. Barnes J. J. Srinivasan P. Whitson E. S. Vander Kaaden K. E. Boyce J. W. Experimental Study into the Stability of Whitlockite in Basaltic Magmas [#6042] We have run experiments to evaluate the magmatic stability of the mineral whitlockite in basaltic systems. Our experiments will address the distribution of water between melt apatite and merrillite-whitlockite solid solutions. Kizovski T. V. Tait K. T. Detailed Mineralogy and Petrology of Intermediate Martian Lherzolitic Shergottite Northwest Africa 6342 [#6111] NWA 6342 is a 35.5 g lherzolitic shergottite that was found in Algeria in 2010. The purpose of this work is to complete a detailed mineralogical, petrological, and geochemical analysis of NWA 6342 and compare it to other lherzolitic sherogottites. Rahib R. R. Udry A. Howarth G. H. Constraining Formation and Emplacement Processes of Poikilitic Shergottites Using Quantitative Textural Analyses [#6063] Quantitative textural analyses of poikilitic shergottites, a relatively rare and understudied group of martian meteorites, will provide insight about their formation and emplacement on Mars. Mari N. Hallis L. J. Riches A. J. V. Lee M. R. Mg-Fe and P-Zoning in Tissint Olivine and Pyroxene: Implications for Martian Magma Chamber Dynamics [#6012] In this work we report major element core-to-rim evolution of pyroxene and P-zoning in olivine in Tissint in order to provide new insights into the dynamics of its parental magma chamber. Mari N. -

Noble Gas Distribution in the Yamato 000593 Nakhlite Deciphered By
40th Lunar and Planetary Science Conference (2009) 1682.pdf NOBLE GAS DISTRIBUTIONS IN THE YAMATO 000593 NAKHLITE DECIPHERED BY LASER ABLATION ANALYSIS AND MINERAL SEPARATION. K. Nagao1, J. Park2, R. Okazaki3, N. Imae4 and H. Kojima4. 1Laboratory for Earthquake Chemistry, Graduate School of Science, University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan ([email protected]), 2ARES, NASA, Johnson Space Center, Houston, TX 77058, USA, 3Department of Earth and Planetary Sciences, Faculty of Sciences, Kyushu University, Hakozaki, Fukuoka 812-8581, Japan, 4Antarctic Meteorite Research Center, National Institute of Polar Research, Kaga 1-chome, Itabashi-ku, Tokyo 173-8515, Japan. Introduction: Three meteorites Yamato (Y) steps from 400 to 1800ºC) was applied to the mineral 000593 (13.7 kg), Y000749 (1.3 kg) and Y000802 (22 separates; olivine (75.5 mg), plagioclase (18.1 mg), g) were discovered in Yamato ice field in Antarctica and pyroxene (187.9 mg). However, noble gases ex- by the Japanese Antarctic Research Expedition in 2000 tracted from olivine and plagioclase at 1800ºC gave (JARE-41). They were recognized as paired nakhlites meaningless results due to small amounts of extracted from view points of petrological and mineralogical noble gases, which were comparable with blank levels. observations as well as recovered locations [1]. For noble gas extraction by laser microprobe (Nd- Nakhlites are cumulates [2] and consist of mostly YAG pulse laser, 1064 nm), a meteorite plate with magnesian augite, microcrystalline mesostasis and ~300 µm thick was used. Optical microprobe and smaller amounts of Fe-rich olivine. Parent magma for Scanning electron microprobe observations on the nakhlites may be different from shergottites, because polished surface of the plate showed abundant nakhlites have high volatile contents, in contrast to mesostasis, probably weathering products, while id- shergottites, but are LREE-enriched [3].