Advanced Blood Cell Identification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educational Commentary – Blood Cell Id: Peripheral Blood Findings in a Case of Pelger Huët Anomaly
EDUCATIONAL COMMENTARY – BLOOD CELL ID: PERIPHERAL BLOOD FINDINGS IN A CASE OF PELGER HUËT ANOMALY Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits, click on Earn CE Credits under Continuing Education on the left side of the screen. To view the blood cell images in more detail, click on the sample identification numbers underlined in the paragraphs below. This will open a virtual image of the selected cell and the surrounding fields. If the image opens in the same window as the commentary, saving the commentary PDF and opening it outside your browser will allow you to switch between the commentary and the images more easily. Click on this link for the API ImageViewerTM Instructions. Learning Outcomes On completion of this exercise, the participant should be able to • discuss the morphologic features of normal peripheral blood leukocytes; • describe characteristic morphologic findings in Pelger-Huët cells; and • differentiate Pelger-Huët cells from other neutrophils in a peripheral blood smear. Case History: A 30 year old female had a routine CBC performed as part of a physical examination. Her CBC results are as follows: WBC=5.9 x 109/L, RBC=4.53 x 1012/L, Hgb=13.6 g/dL, Hct=40%, MCV=88.3 fL, MCH=29.7 pg, MCHC=32.9 g/dL, Platelet=184 x 109/L. Introduction The images presented in this testing event represent normal white blood cells as well as several types of neutrophils that may be seen in the peripheral blood when a patient has the Pelger-Huët anomaly. -

Correlation of Blood Culture and Band Cell Ratio in Neonatal Septicaemia
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 13, Issue 3 Ver. VI. (Mar. 2014), PP 55-58 www.iosrjournals.org Correlation of blood culture and band cell ratio in neonatal septicaemia. Nautiyal S1., *Kataria V. K1., Pahuja V. K1., Jauhari S1., Roy R. C.,1 Aggarwal B.2 1Department of Microbiology, SGRRIM&HS and SMIH, Dehradun, Uttarakhand, India. 2Department of Paediatrics, SGRRIM&HS and SMIH, Dehradun, Uttarakhand, India. Abstract: Background: Neonatal sepsis is a clinical syndrome characterized by signs and symptoms of infection with or without accompanying bacteraemia in the first month of life. Incidence differs among hospitals depending on variety of factors. Blood culture is considered gold standard for the diagnosis, but does not give a rapid result. Hence, there is a need to look for a surrogate marker for diagnosing neonatal septicaemia. Material & Methods: 335 neonates were studied for clinically suspected septicaemia over a period of one year. Blood was cultured and organism identified biochemically. Parameters of subjects like EOS, LOS and Band cell counts were recorded. Results analysed statistically. Results: Male preponderance was observed. Majority of the cases had a normal vaginal delivery. 47.46% cases had early onset septicaemia. Meconium stained liquor was the predominant risk factor .Culture positivity was found to be 32.24% and 87.96% of them also had band cells percentage ranging from 0 to >25. Conclusion: Band cell count can be used as a surrogate marker for neonatal septicaemia. An upsurge of Candida species as a causative agent in Neonatal septicaemia has been observed. -

BLOOD CELL IDENTIFICATION Educational Commentary Is
EDUCATIONAL COMMENTARY – BLOOD CELL IDENTIFICATION Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on the left side of the screen. Learning Outcomes After completion of this exercise, the participant will be able to: • identify morphologic features of normal peripheral blood leukocytes and platelets. • describe characteristic morphologic findings associated with reactive lymphocytes. • compare morphologic features of normal lymphocytes, reactive lymphocytes, and monocytes. Photograph BCI-01 shows a reactive lymphocyte. The term “variant” is also used to describe these cells that display morphologic characteristics different from what is considered normal lymphocyte appearance. Reactive lymphocytes demonstrate a wide variety of morphologic features. They are most often associated with viral illnesses, so it is expected that some of these cells would be present in the peripheral blood of this patient. This patient had infectious mononucleosis that was confirmed with a positive mononucleosis screening test. An increased number of reactive lymphocytes is a morphologic hallmark of infectious mononucleosis. Some generalizations regarding the morphology of reactive lymphocytes can be made. These cells are often large with abundant cytoplasm. Cytoplasmic vacuoles and/or azurophilic granules may also be present. Reactive lymphocytes have an increased amount of RNA in the cytoplasm, which is reflected by an associated increase in cytoplasmic basophilia. The cytoplasm may stain gray, pale-blue, or a very deep blue and appear patchy. The cytoplasmic margins may be indented by surrounding red blood cells and appear a darker blue than the rest of the cytoplasm. Likewise, the nuclei in reactive lymphocytes are variably shaped and may be round, oval, indented, or lobulated. -

Plasma Cells: Finding New Light at the End of B Cell Development Kathryn L
© 2001 Nature Publishing Group http://immunol.nature.com REVIEW Plasma cells: finding new light at the end of B cell development Kathryn L. Calame Plasma cells are cellular factories devoted entire- Upon plasma cell differentiation, there is a marked increase in ly to the manufacture and export of a single prod- steady-state amounts of Ig heavy and light chain mRNA and, when 2 uct: soluble immunoglobulin (Ig). As the final required for IgM and IgA secretion, J chain mRNA . Whether the increase in Ig mRNA is due to increased transcription, increased mediators of a humoral response, plasma cells mRNA stability or, as seems likely, both mechanisms, remains con- play a critical role in adaptive immunity.Although troversial2. There is also an increase in secreted versus membrane intense effort has been devoted to studying the forms of heavy chain mRNA, as determined by differential use of poly(A) sites that may involve the availability of one component of regulation and requirements for early B cell the polyadenylation machinery, cleavage-stimulation factor Cst-643. development, little information has been avail- To accommodate translation and secretion of the abundant Ig able on plasma cells. However, more recent mRNAs, plasma cells have an increased cytoplasmic to nuclear ratio work—including studies on genetically altered and prominent amounts of rough endoplasmic reticulum and secreto- ry vacuoles. mice and data from microarray analyses—has Numerous B cell–specific surface proteins are down-regulated begun to identify the regulatory cascades that upon plasma cell differentiation, including major histocompatibility initiate and maintain the plasma cell phenotype. complex (MHC) class II, B220, CD19, CD21 and CD22. -

REVIEW Multiple Myeloma: the Cells of Origin – a Two-Way Street
Leukemia (1998) 12, 121–127 1998 Stockton Press All rights reserved 0887-6924/98 $12.00 REVIEW Multiple myeloma: the cells of origin – A two-way street JR Berenson, RA Vescio and J Said West Los Angeles VA Medical Center, UCLA School of Medicine, Los Angeles, CA, USA Multiple myeloma results from an interplay between the mono- earlier precursor cells may be responsible for the proliferation clonal malignant plasma cells and supporting nonmalignant of the malignant population. The presence of a circulating cells in the bone marrow. Recent studies suggest that the final transforming event in this B cell disorder occurs at a late stage tumor component without obvious plasma cell morphology of B cell differentiation based on the characteristics of the also suggests that less mature lymphocytes may be part of the immunoglobulin genes expressed by the malignant clone as clone as well, which could explain the dissemination of the well as surface markers present on the tumor cells. Recently, disease throughout the bone marrow.4 In addition, evidence an increasing pathogenic role in this malignancy by the nonma- for tumor cells at even earlier stages of hematopoietic differen- lignant cells in the bone marrow has been suggested by several tiation came from studies showing the high rate of acute non- studies. Specific infection of these supporting cells by the lymphoblastic leukemia in these patients and the presence of recently identified Kaposi’s sarcoma-associated herpes virus 5,6 (KSHV) suggests a novel mechanism by which this nonmalig- non-lymphoid surface markers on malignant plasma cells. A nant population may lead to the development of this B cell variety of molecular biological techniques have subsequently malignancy and support its growth. -

Heterogeneity of Polymorphonuclear Neutrophils in HIV-1 Infection. Study of SIV-Infected Cynomolgus Macaque Model
Heterogeneity of polymorphonuclear neutrophils in HIV-1 infection. Study of SIV-infected cynomolgus macaque model. Julien Lemaitre To cite this version: Julien Lemaitre. Heterogeneity of polymorphonuclear neutrophils in HIV-1 infection. Study of SIV- infected cynomolgus macaque model.. Innate immunity. Université Paris Saclay (COmUE), 2019. English. NNT : 2019SACLS267. tel-02955513 HAL Id: tel-02955513 https://tel.archives-ouvertes.fr/tel-02955513 Submitted on 2 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Heterogeneity of polymorphonuclear neutrophils in HIV-1 infection Study of SIV-infected cynomolgus 2019SACLS267 macaque model : NNT Thèse de doctorat de l'Université Paris-Saclay préparée à l’Université Paris-Sud École doctorale n°569 Innovation thérapeutique : du fondamental à l’appliqué (ITFA) Spécialité : Immunologie Thèse présentée et soutenue à Fontenay-aux-Roses, le 13 septembre 2019, par Monsieur Julien Lemaitre Composition du Jury : Sylvie Chollet-Martin Professeur–Praticien hospitalier, GHPNVS-INSERM (UMR-996) Présidente Véronique -

Restoring Natural Killer Cell Immunity Against Multiple Myeloma in the Era of New Drugs
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Institutional Research Information System University of Turin Restoring Natural Killer Cell immunity against Multiple Myeloma in the era of New Drugs Gianfranco Pittari1, Luca Vago2,3, Moreno Festuccia4,5, Chiara Bonini6,7, Deena Mudawi1, Luisa Giaccone4,5 and Benedetto Bruno4,5* 1 Department of Medical Oncology, National Center for Cancer Care and Research, HMC, Doha, Qatar, 2 Unit of Immunogenetics, Leukemia Genomics and Immunobiology, IRCCS San Raffaele Scientific Institute, Milano, Italy, 3 Hematology and Bone Marrow Transplantation Unit, IRCCS San Raffaele Scientific Institute, Milano, Italy, 4 Department of Oncology/Hematology, A.O.U. Città della Salute e della Scienza di Torino, Presidio Molinette, Torino, Italy, 5 Department of Molecular Biotechnology and Health Sciences, University of Torino, Torino, Italy, 6 Experimental Hematology Unit, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milano, Italy, 7 Vita-Salute San Raffaele University, Milano, Italy Transformed plasma cells in multiple myeloma (MM) are susceptible to natural killer (NK) cell-mediated killing via engagement of tumor ligands for NK activating receptors or “missing-self” recognition. Similar to other cancers, MM targets may elude NK cell immunosurveillance by reprogramming tumor microenvironment and editing cell surface antigen repertoire. Along disease continuum, these effects collectively result in a pro- gressive decline of NK cell immunity, a phenomenon increasingly recognized as a critical determinant of MM progression. In recent years, unprecedented efforts in drug devel- opment and experimental research have brought about emergence of novel therapeutic interventions with the potential to override MM-induced NK cell immunosuppression. -

B-Cell Development, Activation, and Differentiation
B-Cell Development, Activation, and Differentiation Sarah Holstein, MD, PhD Nov 13, 2014 Lymphoid tissues • Primary – Bone marrow – Thymus • Secondary – Lymph nodes – Spleen – Tonsils – Lymphoid tissue within GI and respiratory tracts Overview of B cell development • B cells are generated in the bone marrow • Takes 1-2 weeks to develop from hematopoietic stem cells to mature B cells • Sequence of expression of cell surface receptor and adhesion molecules which allows for differentiation of B cells, proliferation at various stages, and movement within the bone marrow microenvironment • Immature B cell leaves the bone marrow and undergoes further differentiation • Immune system must create a repertoire of receptors capable of recognizing a large array of antigens while at the same time eliminating self-reactive B cells Overview of B cell development • Early B cell development constitutes the steps that lead to B cell commitment and expression of surface immunoglobulin, production of mature B cells • Mature B cells leave the bone marrow and migrate to secondary lymphoid tissues • B cells then interact with exogenous antigen and/or T helper cells = antigen- dependent phase Overview of B cells Hematopoiesis • Hematopoietic stem cells (HSCs) source of all blood cells • Blood-forming cells first found in the yolk sac (primarily primitive rbc production) • HSCs arise in distal aorta ~3-4 weeks • HSCs migrate to the liver (primary site of hematopoiesis after 6 wks gestation) • Bone marrow hematopoiesis starts ~5 months of gestation Role of bone -

Blood Cells and Hemopoiesis IUSM – 2016
Lab 8 – Blood Cells and Hemopoiesis IUSM – 2016 I. Introduction Blood Cells and Hemopoiesis II. Learning Objectives III. Keywords IV. Slides A. Blood Cells 1. Erythrocytes (Red Blood Cells) 2. Leukocytes (White Blood Cells) a. Granulocytes (PMNs) i. Neutrophils ii. Eosinophils iii. Basophils b. Agranulocytes (Mononuclear) i. Lymphocytes ii. Monocytes 3. Thrombocytes (Platelets) B. Bone Marrow 1. General structure 2. Cells a. Megakaryocytes b. Hemopoietic cells i. Erythroid precursors ii. Myeloid precursors V. Summary SEM of a neutrophil (purple) ingesting S. aureus bacteria (yellow). NIAID. Lab 8 – Blood Cells and Hemopoiesis IUSM – 2016 Blood I. Introduction II. Learning Objectives 1. Blood is a specialized type of fluid connective III. Keywords tissue that provides the body’s tissues with IV. Slides nutrition, oxygen, and waste removal and serves A. Blood Cells as a means of transportation for the activity of 1. Erythrocytes (Red Blood Cells) other body systems (e.g., carrying hormones 2. Leukocytes (White Blood Cells) from source to target for the endocrine system). a. Granulocytes (PMNs) 2. It consists of plasma (liquid ECM of blood) and i. Neutrophils formed elements (cells and platelets). ii. Eosinophils iii. Basophils 3. The “formed elements” of blood derive from b. Agranulocytes (Mononuclear) hematopoietic stem cells located in the red bone i. Lymphocytes marrow of flat bones in adults. ii. Monocytes 4. Blood cells can be classified as red blood cells 3. Thrombocytes (Platelets) (about 45% of blood) and white blood cells B. Bone Marrow (about 1% of blood) based upon their gross 1. General structure appearance upon centrifugation. 2. Cells a. Megakaryocytes 5. -

Distribution in Trout Immune Tissues Plasmablast and Plasma Cell
The Journal of Immunology Plasmablast and Plasma Cell Production and Distribution in Trout Immune Tissues1 Erin S. Bromage,* Ilsa M. Kaattari,* Patty Zwollo,† and Stephen L. Kaattari2* These studies describe the in vitro and ex vivo generation of plasmablasts and plasma cells in trout (Oncorhynchus mykiss) peripheral blood and splenic and anterior kidney tissues. Cells were derived either from naive trout and cultured with the polyclonal activator, Escherichia coli LPS, or from trout that had been immunized with trinitrophenyl-keyhole limpet hemocyanin. Hydroxyurea was used to resolve populations of replicating (plasmablast) and nonreplicating (plasma cell) Ab-secreting cells (ASC). Complete inhibition of Ig secretion was only observed within the PBL. Both anterior kidney and splenic lymphocytes possessed a subset of ASCs that were hydroxyurea resistant. Thus, in vitro production of plasma cells appears to be restricted to the latter two tissues, whereas peripheral blood is exclusively restricted to the production of plasmablasts. After immunization with trinitrophenyl-keyhole limpet hemocyanin, specific ASC could be isolated from all immune organs; however, the anterior kidney contained 98% of all ASC. Late in the response (>10 wk), anterior kidney ASC secreted specific Ab for at least 15 days in culture, indicating that they were long-lived plasma cells. Cells from spleen and peripheral blood lost all capacity to secrete specific Ab in the absence of Ag. Late in the Ab response, high serum titer levels are solely the result of Ig secretion from anterior kidney plasma cells. The Journal of Immunology, 2004, 173: 7317–7323. he immune system of the trout and other teleosts exhibits Plasma cells may become long-lived upon migration to a sup- two striking anatomical differences from mammals: 1) portive niche within the bone marrow, relying on specialized cues T they possess neither bone marrow nor lymph nodes; and for their longevity (14). -
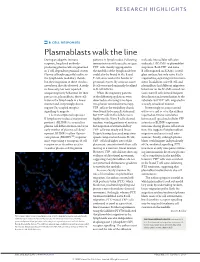
B Cell Responses: Plasmablasts Walk the Line
RESEARCH HIGHLIGHTS B CELL RESPONSES Plasmablasts walk the line During an adaptive immune patterns in lymph nodes. Following molecule intercellular adhesion response, long-lived antibody- immunization with specific antigen, molecule 1 (ICAM1) in plasmablast producing plasma cells are generated YFP+ cells mainly aggregated in migration. Both YFP+ and naive in T cell-dependent germinal centres. the medulla of the lymph node but B cells migrated on ICAM1-coated Plasma cells subsequently localize to could also be found in the B and glass surfaces, but only naive B cells the lymph node medullary chords, T cell zones and at the border of required Gαi-signalling for this move- but their migration to these sites has germinal centres. By contrast, naive ment. In addition, naive B cells and never been directly observed. A study B cells were predominantly localized plasma blasts had different migratory in Immunity has now reported in B cell follicles. behaviour on the ICAM1-coated sur- unique migratory behaviour for their When the migratory patterns faces; naive B cells showed frequent precursors, plasmablasts; these cells of the different populations were detachment and reattachment to the traverse the lymph node in a linear observed in situ using time-lapse substrate, but YFP+ cells migrated in manner and, surprisingly, do not two-photon intravital microscopy, a steady, amoeboid manner. + require Gαi-coupled receptor YFP cells in the medullary chords Interestingly, in assays carried signalling to migrate. were found to be mainly stationary, out in vivo and in vitro, the authors The transcriptional repressor but YFP+ cells in the follicles were reported an inverse correlation B lymphocyte-induced maturation highly motile. -

Lymphoid System IUSM – 2016
Lab 14 – Lymphoid System IUSM – 2016 I. Introduction Lymphoid System II. Learning Objectives III. Keywords IV. Slides A. Thymus 1. General Structure 2. Cortex 3. Medulla B. Lymph Nodes 1. General Structures 2. Cortex 3. Paracortex 4. Medulla C. MALT 1. Tonsils 2. BALT 3. GALT a. Peyer’s patches b. Vermiform appendix D. Spleen 1. General Structure 2. White Pulp 3. Red Pulp V. Summary SEM of an activated macrophage. Lab 14 – Lymphoid System IUSM – 2016 I. Introduction Introduction II. Learning Objectives III. Keywords 1. The main function of the immune system is to protect the body against aberrancy: IV. Slides either foreign pathogens (e.g., bacteria, viruses, and parasites) or abnormal host cells (e.g., cancerous cells). A. Thymus 1. General Structure 2. The lymphoid system includes all cells, tissues, and organs in the body that contain 2. Cortex aggregates (accumulations) of lymphocytes (a category of leukocytes including B-cells, 3. Medulla T-cells, and natural-killer cells); while the functions of the different types of B. Lymph Nodes lymphocytes vary greatly, they generally all appear morphologically similar so cannot be 1. General Structures routinely distinguished in light microscopy. 2. Cortex 3. Lymphocytes can be found distributed throughout the lymphoid system as: (1) single 3. Paracortex cells, (2) isolated aggregates of cells, (3) distinct non-encapsulated lymphoid nodules in 4. Medulla loose CT associated with epithelium, or (4) encapsulated individual lymphoid organs. C. MALT 1. Tonsils 4. Primary lymphoid organs are sites where lymphocytes are formed and mature; they 2. BALT include the bone marrow (B-cells) and thymus (T-cells); secondary lymphoid organs are sites of lymphocyte monitoring and activation; they include lymph nodes, MALT, and 3.