Supplemental Data for Drug Metabolism and Disposition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

( 12 ) United States Patent
US010376507B2 (12 ) United States Patent (10 ) Patent No. : US 10 , 376 , 507 B2 Srinivasan et al. (45 ) Date of Patent: Aug. 13 , 2019 CRESEMBA® ( isavuconazonium sulfate ) , Highlights of Prescrib (54 ) METHOD OF TREATING A PATIENT WITH ing Information , Label ; Patient Information approved by the U . S . A CYP3A4 SUBSTRATE DRUG Food and Drug Administration ; Astellas Pharma US , Inc. ( Licensed from Basilea Pharmaceutics International Ltd . ) , Illinois, USA , Ini (71 ) Applicant: Bow River LLC , Corona Del Mar , CA tial U . S . Approval: 2015 , Revised Mar. 2015 , Reference ID : 3712237, ( US ) 28 pages . DIFLUCAN® ( fluconazole ), Label ; Patient Information , Reference ( 72 ) Inventors : Sundar Srinivasan , Corona Del Mar, ID : 3650838 , Roerig , Division of Pfizer Inc ., New York , NY, Revised Mar. 2013 , 35 pages. CA (US ) ; Christina Chow , Seattle , WA NIZORAL® (ketoconazole )Label ; Patient Information approved by (US ) the U . S . Food and Drug Administration , Reference ID : 3458324 , Copyright 2014 Janssen Pharmaceuticals, Inc . , New Jersey, USA , ( 73 ) Assignee : BOW RIVER LLC , Corona del Mar , Revised Feb . 2014 , 23 pages . NOXAFIL® (posaconazole ) , Highlights of Prescribing Informa CA (US ) tion , Label ; Patient Information approved by the U . S . Food and Drug Administration ; Copyright © 2006 , 2010 , 2013 , 2014 Merck ( * ) Notice : Subject to any disclaimer , the term of this Sharp & Dohme Corp . , a subsidiary of Merck & Co ., Inc ., New patent is extended or adjusted under 35 Jersey , USA , Revised Sep . 2016 , Reference ID : 3983525, 37 pages . U . S . C . 154 (b ) by 0 days. ORAVIG® (miconazole ) , Highlights of Prescribing Information , Label ; Patient Information approved by the U . S . Food and Drug Administration ; Copyright 2012 Praelia Pharmaceuticals , Inc . , North (21 ) Appl. -

Addyi Generic Name: Flibanserin Manufacturer
Brand Name: Addyi Generic Name: Flibanserin Manufacturer: Sprout Pharmaceuticals Drug Class: Central Nervous System Agent, Serotonin Agonist, Dopamine antagonist Uses: Labeled Uses: Indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD) as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to: A co-existing medical or psychiatric condition, problems within the relationship, or the effects of a medication or other drug substance. Unlabeled Uses: none. Mechanism of Action: The mechanism of action for flibanserin in the treatment of hypoactive sexual desire disorder is unknown. Flibanserin has high affinity for serotonin (5-hydroxytryptamine or 5-HT) 1A receptors, as an agonist, and 5-HT2A receptors, as an antagonist, and moderate affinity for 5- HT2B, 5-HT2C, and dopamine D4 receptors as an antagonist Pharmacokinetics: Absorption: Tmax 0.75 hours Vd 50L t ½ 11 hours Clearance Not reported Protein binding 98% (albumin) Bioavailability 33% Metabolism: Flibanserin is extensively metabolized primarily by CYP3A4 and, to a lesser extent, CYP2C19 to at least 35 metabolites, with most of the metabolites occurring in low concentrations in plasma. Elimination: Flibanserin is primarily excreted through the kidneys in to urine (44%) and feces (51%). Two metabolites could be characterized that showed plasma concentration similar to that achieved with flibanserin: 6,21-dihydroxy-flibanserin-6,21-disulfate and 6- hydroxy-flibanserin-6-sulfate. These two metabolites are inactive. Efficacy: Katz M, DeRogatis LR, Ackerman R, et al. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. -
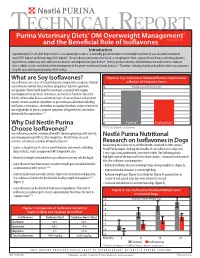
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

Applications of in Silico Methods to Analyze the Toxicity and Estrogen T Receptor-Mediated Properties of Plant-Derived Phytochemicals ∗ K
Food and Chemical Toxicology 125 (2019) 361–369 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Applications of in silico methods to analyze the toxicity and estrogen T receptor-mediated properties of plant-derived phytochemicals ∗ K. Kranthi Kumara, P. Yugandharb, B. Uma Devia, T. Siva Kumara, N. Savithrammab, P. Neerajaa, a Department of Zoology, Sri Venkateswara University, Tirupati, 517502, India b Department of Botany, Sri Venkateswara University, Tirupati, 517502, India ARTICLE INFO ABSTRACT Keywords: A myriad of phytochemicals may have potential to lead toxicity and endocrine disruption effects by interfering Phytochemicals with nuclear hormone receptors. In this examination, the toxicity and estrogen receptor−binding abilities of a QSAR modeling set of 2826 phytochemicals were evaluated. The endpoints mutagenicity, carcinogenicity (both CAESAR and ISS Toxicity models), developmental toxicity, skin sensitization and estrogen receptor relative binding affinity (ER_RBA) Nuclear hormone receptor binding were studied using the VEGA QSAR modeling package. Alongside the predictions, models were providing pos- Self−Organizing maps sible information for applicability domains and most similar compounds as similarity sets from their training Clustering and classification schemes sets. This information was subjected to perform the clustering and classification of chemicals using Self−Organizing Maps. The identified clusters and their respective indicators were considered as potential hotspot structures for the specified data set analysis. Molecular screening interpretations of models wereex- hibited accurate predictions. Moreover, the indication sets were defined significant clusters and cluster in- dicators with probable prediction labels (precision). Accordingly, developed QSAR models showed good pre- dictive abilities and robustness, which observed from applicability domains, representation spaces, clustering and classification schemes. -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -

Grapefruit Juice May Cause Prescription Drug Interactions
Grapefruit Juice May Cause Prescription Drug Interactions Grapefruit juice provides many nutrients, including vitamin C, potassium and lycopene. But chemicals in grapefruit juice and grapefruit pulp interfere with the enzymes that break down (metabolize) various drugs in the digestive system — including certain calcium channel blockers and cholesterol- lowering drugs. The result can be excessively high levels of these drugs in the blood and an increased risk of potentially serious side effects. Pomelos and Seville oranges, a type of bitter orange often used to make marmalade and compotes, may have a similar effect. Here's a sampling of drugs known to have potentially serious interactions with grapefruit products: Drug name Type of drug Amiodarone (Cordarone) A drug used to treat and prevent abnormal heart rhythms (arrhythmias) Buspirone (BuSpar), sertraline (Zoloft) Antidepressants Carbamazepine (Carbatrol, Tegretol) An anti-seizure medication Cyclosporine (Neoral, Sandimmune), tacrolimus Immunosuppressant drugs (Prograf) Felodipine (Plendil), nifedipine (Procardia), Calcium channel blockers used to treat high blood nimodipine (Nimotop), nisoldipine (Sular) pressure Saquinavir An HIV medication Simvastatin (Zocor), lovastatin (Mevacor), Statins used to treat high cholesterol atorvastatin (Lipitor) If you're concerned about the effect grapefruit juice may have on your medications, talk to your doctor or pharmacist. In some cases, it may be important to avoid grapefruit and grapefruit products, as well as pomelos, Seville oranges and products made with these fruits. Waiting to take these medications — even up to 24 hours — after you drink grapefruit juice won't prevent an interaction. In other cases, it may be possible to switch to an alternative medication that won't interact with these fruits. -

(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

Daidzein and Genistein Content of Cereals
European Journal of Clinical Nutrition (2002) 56, 961–966 ß 2002 Nature Publishing Group All rights reserved 0954–3007/02 $25.00 www.nature.com/ejcn ORIGINAL COMMUNICATION Daidzein and genistein content of cereals J Liggins1, A Mulligan1,2, S Runswick1 and SA Bingham1,2* 1Medical Research Council Dunn Human Nutrition Unit, Hills Road, Cambridge, UK; and 2European Prospective Investigation of Cancer, University of Cambridge, Cambridge, UK Objective: To analyse 75 cereals and three soy flours commonly eaten in Europe for the phytoestrogens daidzein and genistein. Design: The phytoestrogens daidzein and genistein were extracted from dried foods, and the two isoflavones quantified after hydrolytic removal of any conjugated carbohydrate. Completeness of extraction and any procedural losses of the isoflavones 0 0 were accounted for using synthetic daidzin (7-O-glucosyl-4 -hydroxyisoflavone) and genistin (7-O-glucosyl-4 5-dihydroxyiso- flavone) as internal standards. Setting: Foods from the Cambridge UK area were purchased, prepared for eating, which included cooking if necessary, and freeze dried. Three stock soy flours were also analysed. Results: Eighteen of the foods assayed contained trace or no detectable daidzein or genistein. The soy flours were rich sources, containing 1639 – 2117 mg=kg. The concentration of the two isoflavones in the remaining foods ranged from 33 to 11 873 mg=kg. Conclusion: These analyses will supply useful information to investigators determining the intake of phytoestrogens in cereal products in order to relate intakes to potential biological activities. Sponsorship: This work was supported by the United Kingdom Medical Research Council, Ministry of Agriculture Fisheries and Food (contract FS2034) and the United States of America Army (contract DAMD 17-97-1-7028). -

Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on Β-Alanine and Isatin
Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin by Robert Philip Colaguori A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Pharmaceutical Sciences University of Toronto © Copyright by Robert Philip Colaguori, 2016 ii Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin Robert Philip Colaguori Master of Science Department of Pharmaceutical Sciences University of Toronto 2016 Abstract Epilepsy is the fourth-most common neurological disorder in the world. Approximately 70% of cases can be controlled with therapeutics, however 30% remain pharmacoresistant. There is no cure for the disorder, and patients affected are subsequently medicated for life. Thus, there is a need to develop compounds that can treat not only the symptoms, but also delay/prevent progression. Previous work resulted in the discovery of NC-2505, a substituted β-alanine with activity against chemically induced seizures. Several N- and α-substituted derivatives of this compound were synthesized and evaluated in the kindling model and 4-AP model of epilepsy. In the kindling model, RC1-080 and RC1-102 were able to decrease the mean seizure score from 5 to 3 in aged mice. RC1-085 decreased the interevent interval by a factor of 2 in the 4-AP model. Future studies are focused on the synthesis of further compounds to gain insight on structure necessary for activity. iii Acknowledgments First and foremost, I would like to thank my supervisor Dr. Donald Weaver for allowing me to join the lab as a graduate student and perform the work ultimately resulting in this thesis. -

Mindy Goldman, MD Clinical Professor Dept
Managing Menopause Medically and Naturally Mindy Goldman, MD Clinical Professor Dept. of Ob/Gyn and Reproductive Sciences Director, Women’s Cancer Care Program, UCSF Breast Care Center and Women’s Health University of California, San Francisco I have nothing to disclose –Mindy Goldman, MD CASE STUDY 50 yr. old G2P2 peri-menopausal woman presents with complaints of significant night sweats interfering with her ability to sleep. She has mild hot flashes during the day. She has never had a bone mineral density test but her mother had a hip fracture at age 62 due to osteoporosis. Her 46 yr. old sister was diagnosed with breast cancer at age 43, treated with lumpectomy and radiation and currently is doing well. There is no other family history of cancer. Questions 1. Would you offer her MHT? 2. If yes, how long would you continue it? 3. If no, what would you offer for alternative treatments? 4. Would your treatment differ if you knew she had underlying heart disease? Is it safe? How long can I take it? What about Mymy Bones?bones? Will it protect my heart? MHT - 2015 What about my brain? Will I get breast cancer? What about my hot flashes? Menopausal Symptoms Hot flashes Night sweats Sleep disturbances Vaginal dryness/Sexual dysfunction Mood disturbances How to Treat Menopausal Symptoms Hormone therapy Alternatives to hormones Complementary and Integrative Techniques Prior to Women’s Health Initiative Hormone therapy primary treatment of menopausal hot flashes Few women would continue hormones past one year By 1990’s well known